Nicotine tablet
A technology of nicotine tablets and tablets, which is applied in pill delivery, pharmaceutical formulations, nervous system diseases, etc., can solve the problems of unrealized smoking simulation, and achieve the effect of promoting the absorption of nicotine into the blood stream
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Preparation of rapidly disintegrating tablets
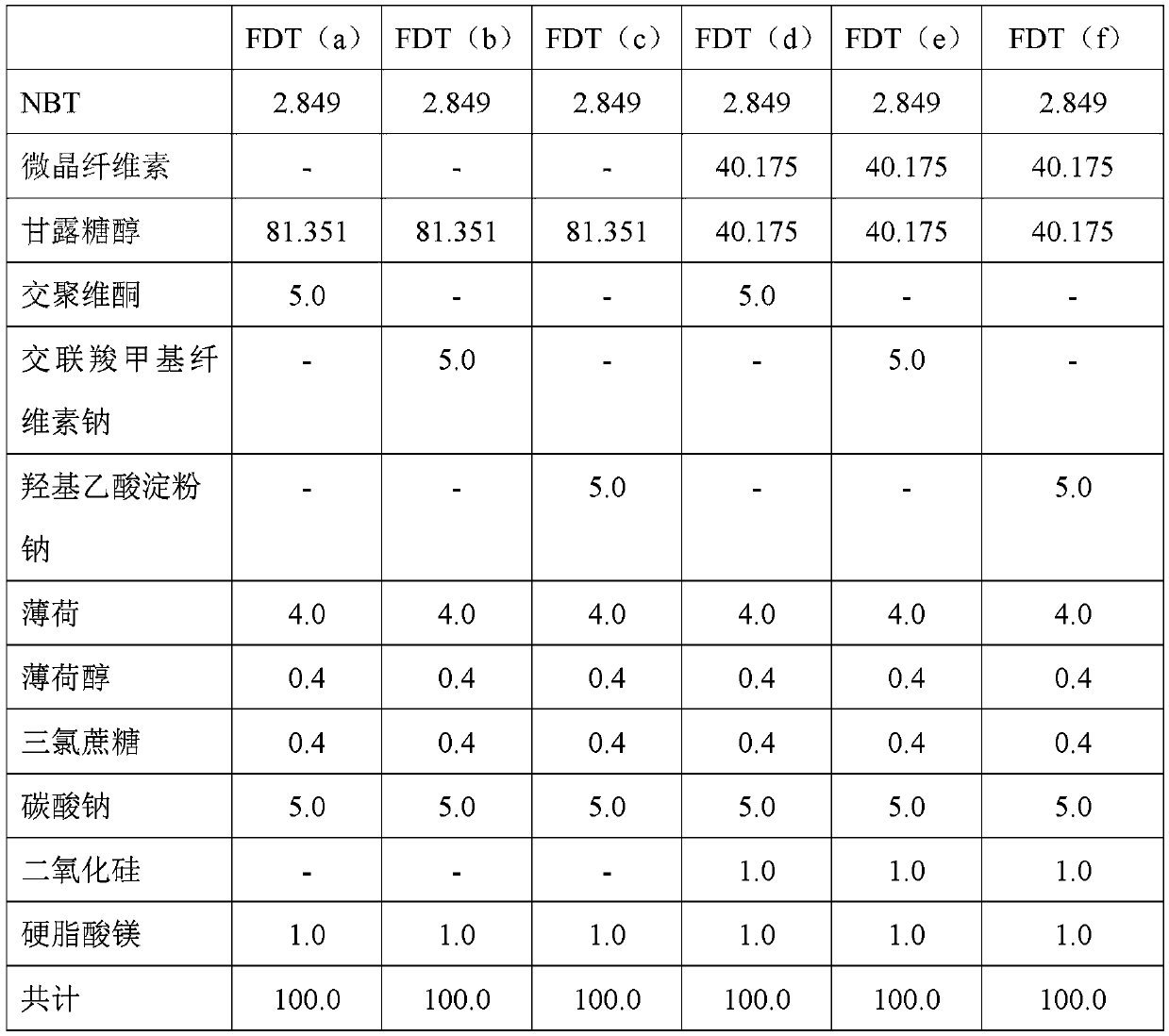
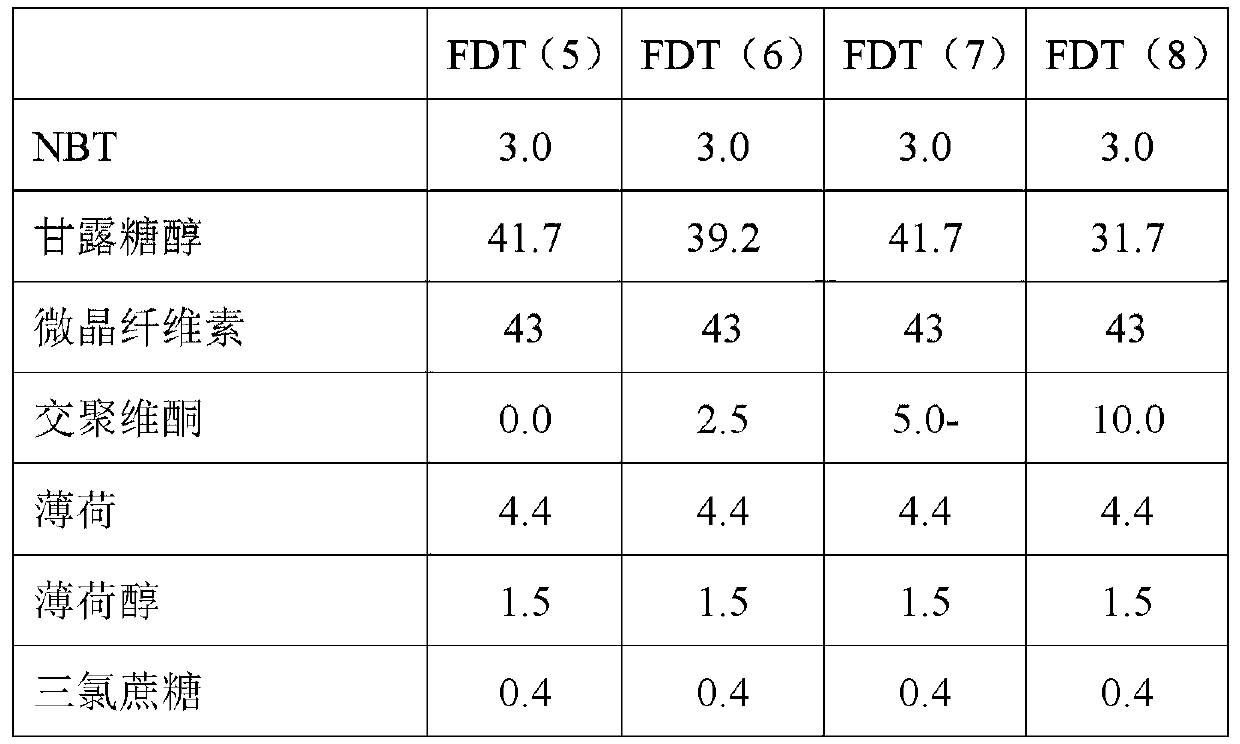
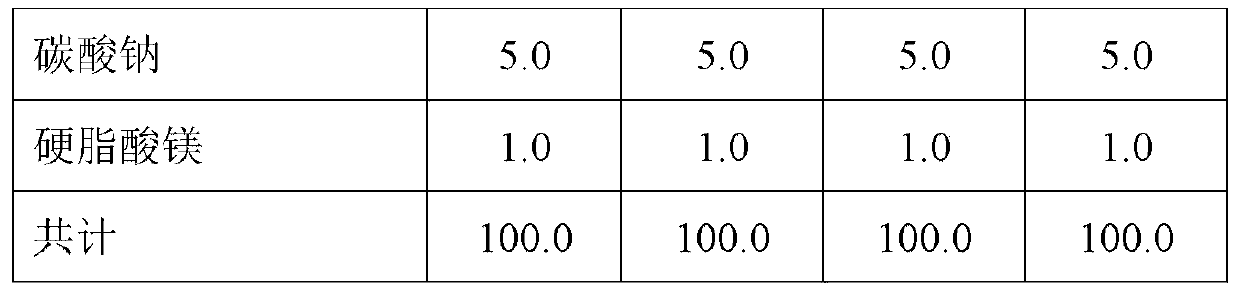
[0128] In this example, six fast disintegrating tablets (FDT) with 1 mg nicotine were prepared into the formulations listed in Table 1. Rapidly disintegrating tablets were prepared with NBT (nicotine bitartrate dihydrate). Punch used: 7.00mm, round, dimpled, D tooling (D tooling). Tablet weight: 100.0mg.
[0129] Table 1 - Rapidly disintegrating tablet compositions. Amounts are given in mg. FDT = Fast Disintegrating Tablet.
[0130]
[0131] Weigh ingredients from bags or drums into individual weighing containers.
[0132] Sieve all excipients through an 800 micron sieve into stainless steel or plastic bins in the following order:
[0133] Half of the bulking / bulk sweetener
[0134] · API and all other excipients, except magnesium stearate
[0135] The remaining half of the bulking / bulk sweetener
[0136] They were mixed in a Turbula mixer at 25 RPM for 4 to 10 minutes. The lubricant (eg, magnesium stearate) i...
Embodiment 2
[0143] Preparation of rapidly disintegrating tablets using a ready-to-use system
[0144] Another way to prepare rapidly disintegrating tablets is to use a ready-to-use system. Suitable for this purpose may be but not limited to: Pearlitol Flash (Roquette), Pharmaburst 500 (SPI Pharma), Ludiflash (BASF), ProSolv (JRS Pharma), ProSolv EasyTab (JRS Pharma), F-Melt (Fuji Chemical), SmartEx50 or SmartEx100 (Shin Etsu / Harke Pharma). These ready-to-use systems are co-processed into a powder mix incorporating fillers, disintegrants, glidants or the like. This saves handling of several excipients and ensures uniformity between excipients.
[0145] In this example, five nicotine-free fast disintegrating tablets (FDT(g) to FDT(k)) were prepared with the ready-to-use system in the formulations listed in Table 3A. Rapidly disintegrating tablets were prepared without NBT (placebo). The addition of nicotine to rapidly disintegrating tablets is expected to have only a small effect on dis...
Embodiment 3
[0215] body pH
[0216] Rapidly disintegrating tablets are designed to have an in vivo pH higher than the resting saliva pH in the oral cavity. Therefore, pH is measured in vivo as follows:
[0217] At least 6 individuals chew the unbuffered gum matrix for 1 minute, after which the initial pH is measured in a sample of saliva from each individual with a suitable pH electrode system (eg stainless steel electrode PHW77-SS). Only individuals with an initial pH in saliva in the range of 6.7 to 7.3 after chewing the unbuffered gum matrix for one minute were selected. These individuals thus qualify as average individuals.
[0218] One tablet is administered sublingually to at least six subjects. Thereafter, the saliva pH of each of the six individuals was measured at specified time intervals. Therefore, each pH value is the arithmetic mean of six measurements made on saliva samples from six individuals.
[0219] The sample volume for each saliva sample may vary since the volume o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com