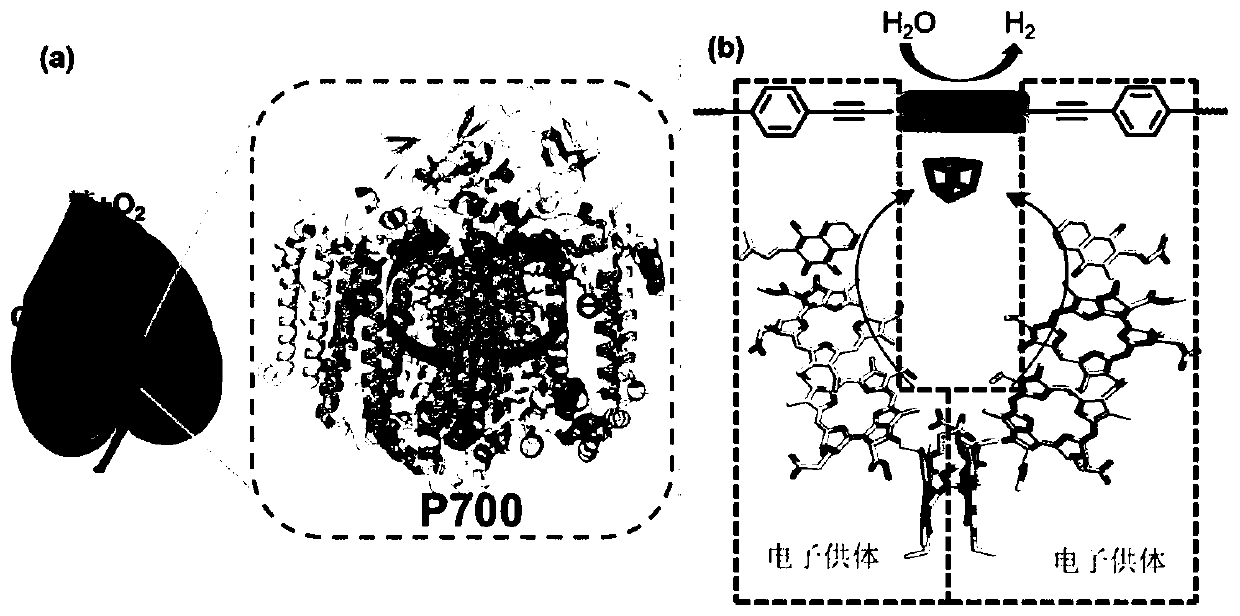
Covalent organic framework material of bionic photosystem I and preparation and application of covalent organic framework material
A covalent organic framework, photosystem technology, applied in organic compound/hydride/coordination complex catalysts, inorganic chemistry, hydrogen production, etc., can solve problems such as biomimetic PSICOFs that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
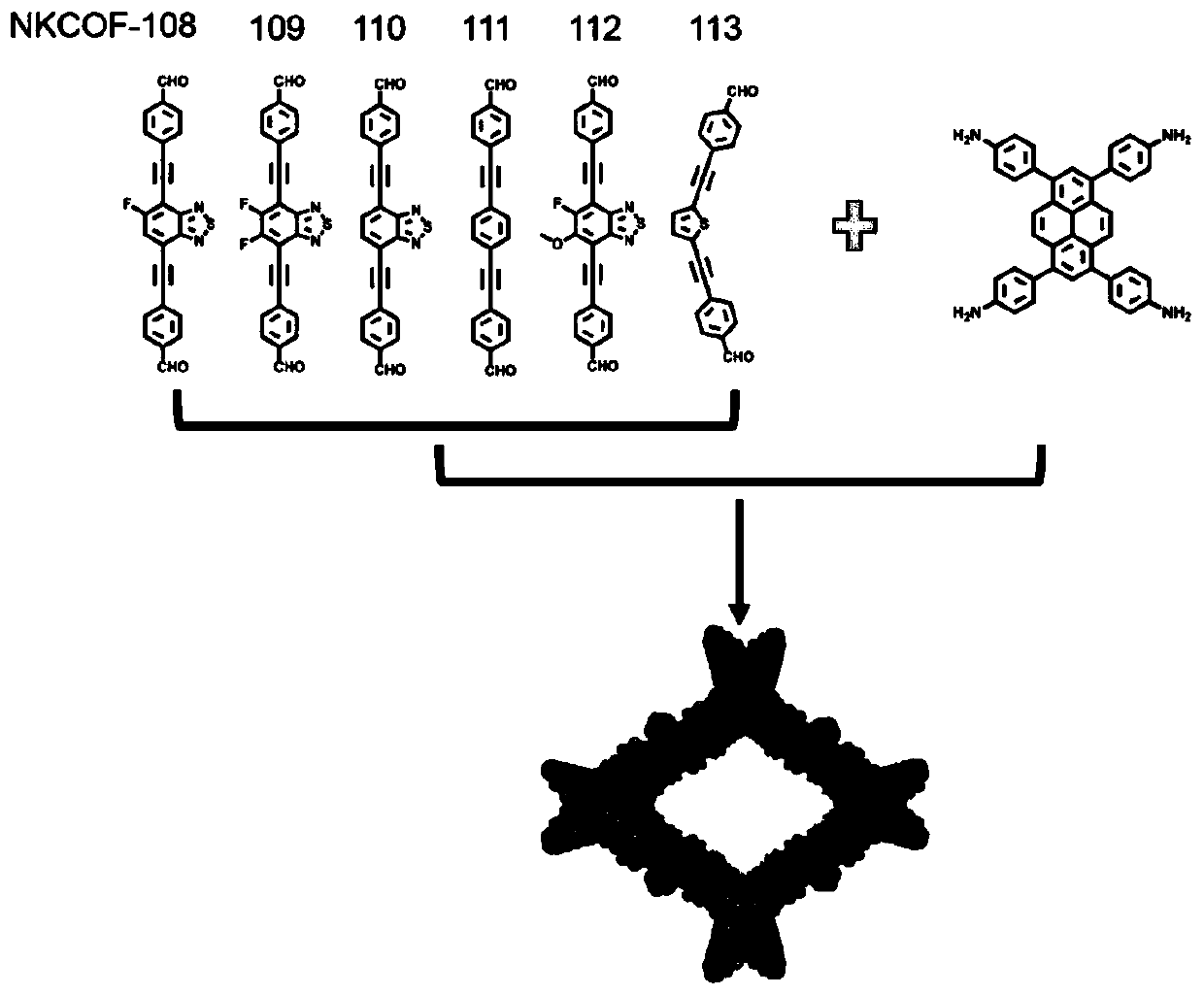
[0039] The synthesis of the covalent organic framework material NKCOF-108 with a biomimetic structure, the specific implementation steps are as follows:
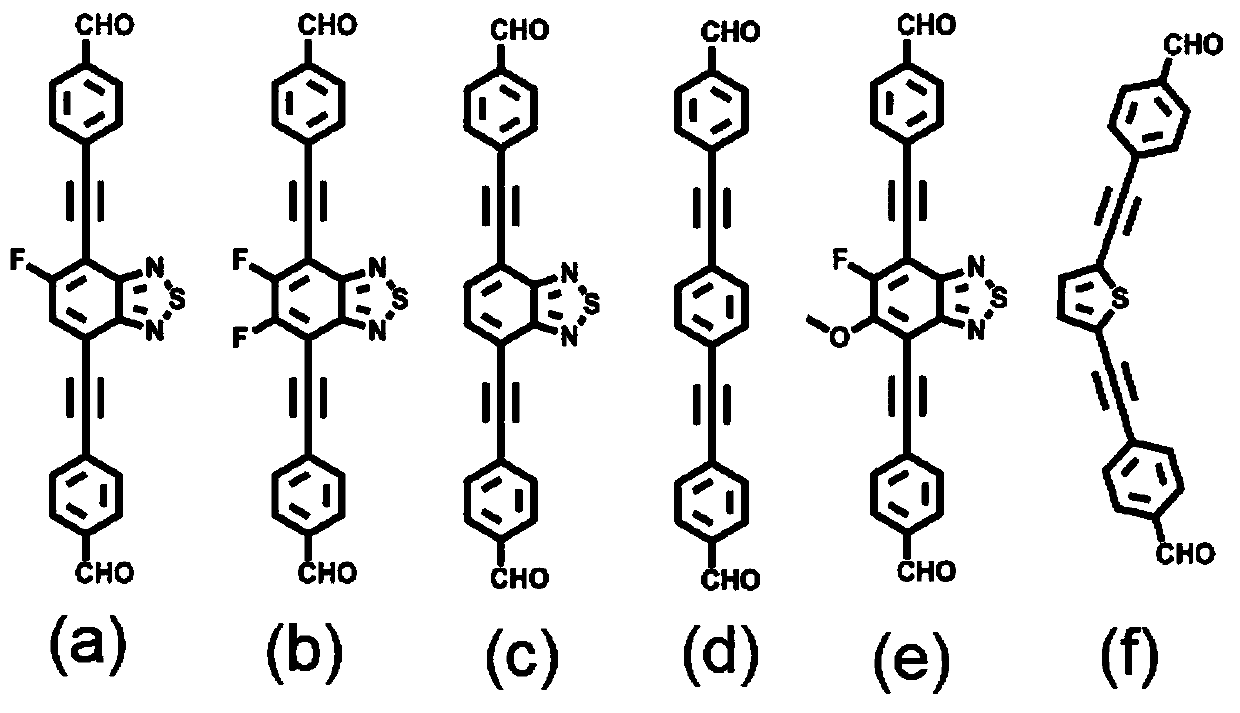
[0040] Weigh the monomer 4,4'-((5-fluorobenzo[c][1,2,5]thiadiazole-4,7-diyl)bis(acetylene-2,1-diyl)) Dibenzaldehyde (0.04mmol) and 1,3,6,8-tetrakis-(p-aminophenyl)-pyrene (0.02mmol) were added into a thick-walled heat-resistant glass tube (o.d.×i.d=10×8mm 2 ), then add 0.95mL of mesitylene, 0.05mL of n-butanol and 0.1mL of 6M acetic acid aqueous solution, then quickly freeze in liquid nitrogen, then vacuumize, and then seal the tube with a flame of an oxygen hydrogen machine. Put the sealed glass tube into an oven at 120°C for 5 days to react to obtain dark red solid product NKCOF-108. Its PXRD as Figure 4 shown.
Embodiment 2
[0042] The synthesis of the covalent organic framework material NKCOF-109 with a biomimetic structure, the specific implementation steps are as follows:
[0043]Weigh the monomer 4,4'-((5,6-difluorobenzo[c][1,2,5]thiadiazole-4,7-diyl)bis(acetylene-2,1-diyl) Base)) benzaldehyde (0.04mmol) and 1,3,6,8-tetra-(p-aminophenyl)-pyrene (0.02mmol) were added into a thick-walled heat-resistant glass tube (o.d.×i.d=10×8mm 2 ), then add 0.1mL mesitylene, 0.9mL n-butanol and 0.1mL 6M acetic acid aqueous solution, then quickly freeze in liquid nitrogen, then vacuumize, and then seal the tube with a flame of an oxygen hydrogen machine. Put the sealed glass tube into an oven at 120°C for 5 days to react to obtain dark red solid product NKCOF-109. Its PXRD as Figure 4 shown.
Embodiment 3
[0045] The synthesis of the covalent organic framework material NKCOF-110 with a biomimetic structure, the specific implementation steps are as follows:
[0046] Weigh the monomer 4,4'-(benzo[c][1,2,5]thiadiazole-4,7-diylbis(ethyn-2,1-diyl))benzaldehyde (0.04 mmol) and 1,3,6,8-tetra-(p-aminophenyl)-pyrene (0.02mmol) into thick-walled heat-resistant glass tube (o.d.×i.d=10×8mm 2 ), then add 0.75mL of mesitylene, 0.25mL of n-butanol and 0.1mL of 6M acetic acid aqueous solution, then quickly freeze in liquid nitrogen, then vacuumize, and then seal the tube with a flame of an oxygen hydrogen machine. Put the sealed glass tube into an oven at 120°C for 5 days to react to obtain dark red solid product NKCOF-110. Its PXRD as Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com