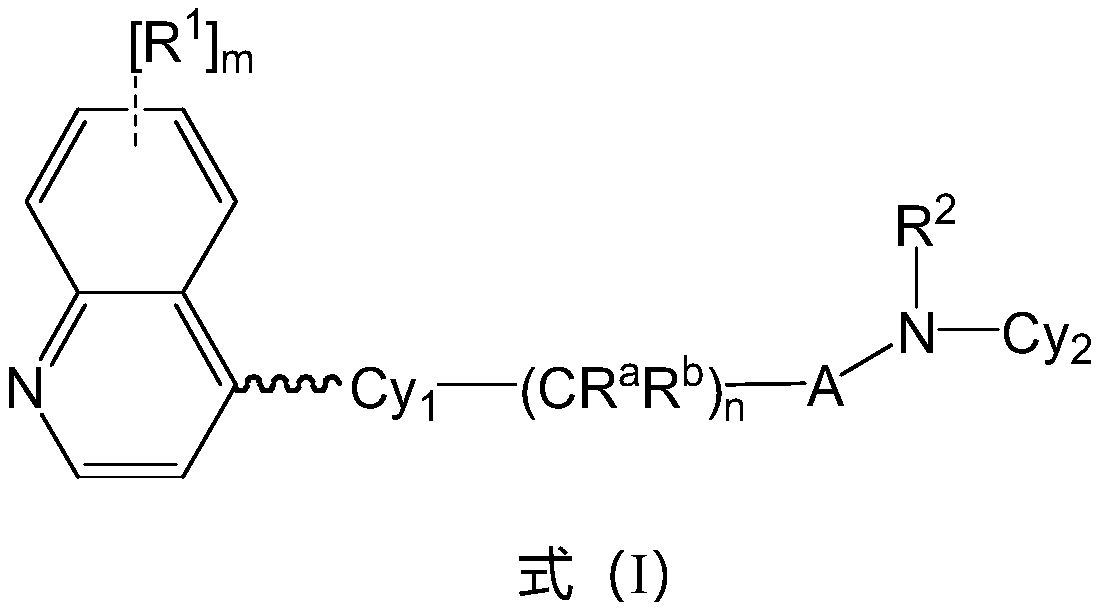
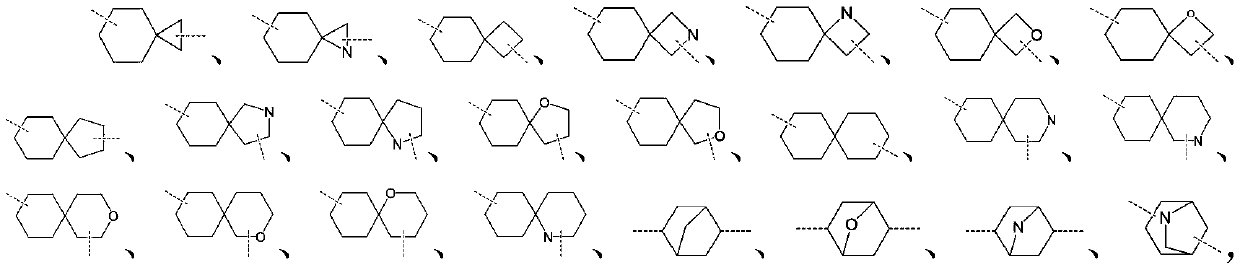
Preparation method of IDO inhibitor
An alkyl and amino technology, applied in the field of preparation of IDO inhibitors, can solve the problems of limited clinical activity, high toxicity, poor biological activity of NLG919, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] N-(4-chlorophenyl)-6-(6-fluoroquinolin-4-yl)spiro[2.5]octane-1-carboxamide
[0094] N-(4-chlorophenyl)-6-(6-fluoroquinolin-4-yl)spiro[2.5]octane-1-carboxamide
[0095]
[0096]The first step: Dissolve 1,4-cyclohexanedione monoethylene glycol ketal (10.0g, 64.03mmol) in 250mL methyl tert-butyl ether, add N-phenylbis(trifluoromethanesulfonyl) Imine (22.9 g, 64.03 mmol). The reaction solution was cooled to -78°C, and sodium bis(trimethylsilyl)amide (2 mol / L tetrahydrofuran solution) (32 mL, 64.03 mmol) was added dropwise to the reaction solution under a nitrogen atmosphere. After the dropwise addition, the reaction solution was stirred at this temperature for 60 minutes, then the reaction solution was raised to room temperature, and stirred overnight until TLC detected that the reaction raw materials were completely consumed. The reaction solution was quenched with 3 mL aqueous potassium bisulfate solution, the solid was removed by filtration, and the filtrate was con...
Embodiment 20
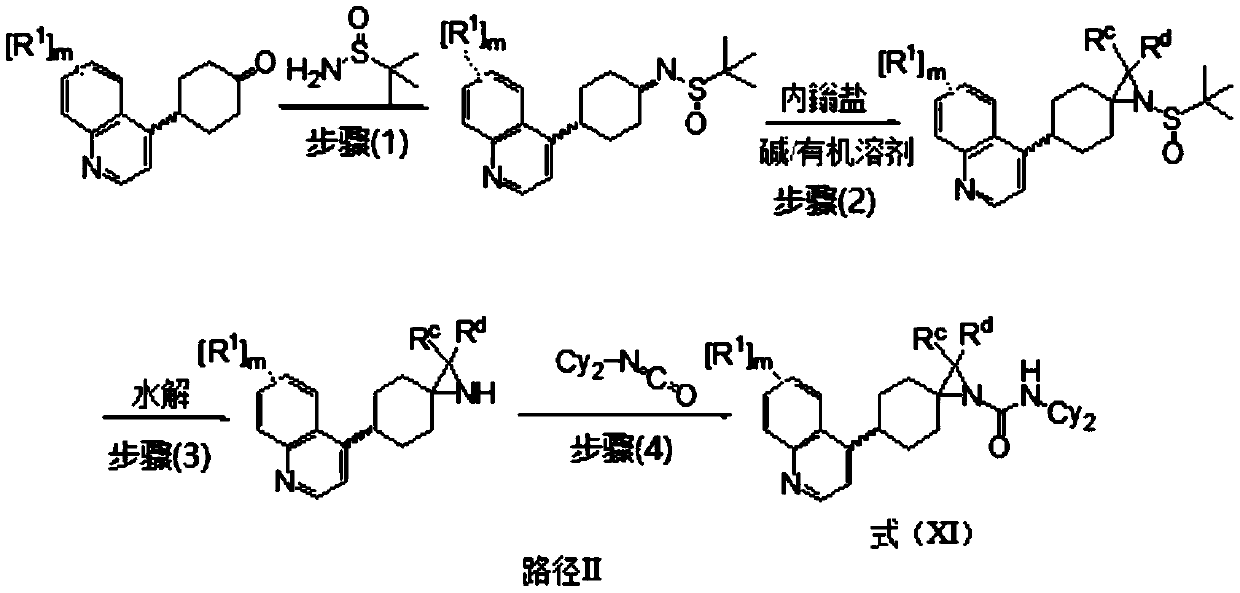
[0105] N-(4-chlorophenyl)-6-(6-fluoroquinolin-4-yl)-1-azaspiro[2.5]octane-1-carboxamide
[0106] N-(4-chlorophenyl)-6-(6-fluoroquinolin-4-yl)-1-azaspiro[2.5]octane-1-carboxamide
[0107]
[0108] The synthesis of compound 20 starts from the intermediate 1e in Example 1 and is prepared via the following steps:
[0109] The first step: Dissolve compound 1e (500mg, 2.06mmol) in 20mL ultra-dry THF, add tetraethoxytitanium (2.81g, 12.36mmol) under nitrogen, and then add tert-butylsulfinamide (747mg, 6.18mmol),. The reaction mixture was reacted at 60°C for 4 hours. The reaction was cooled to room temperature, the mixture was poured into an equal volume of saturated brine and stirred, the resulting suspension was filtered through celite, and the filter cake was washed with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was separated by a fast column machine to obtain compound 20a (51...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com