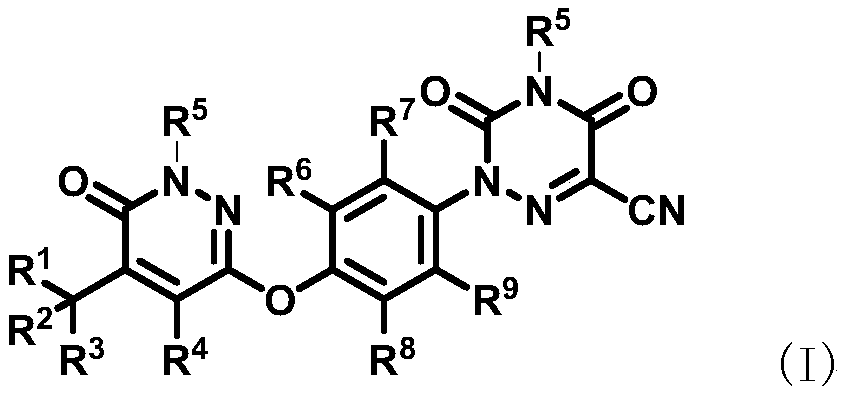
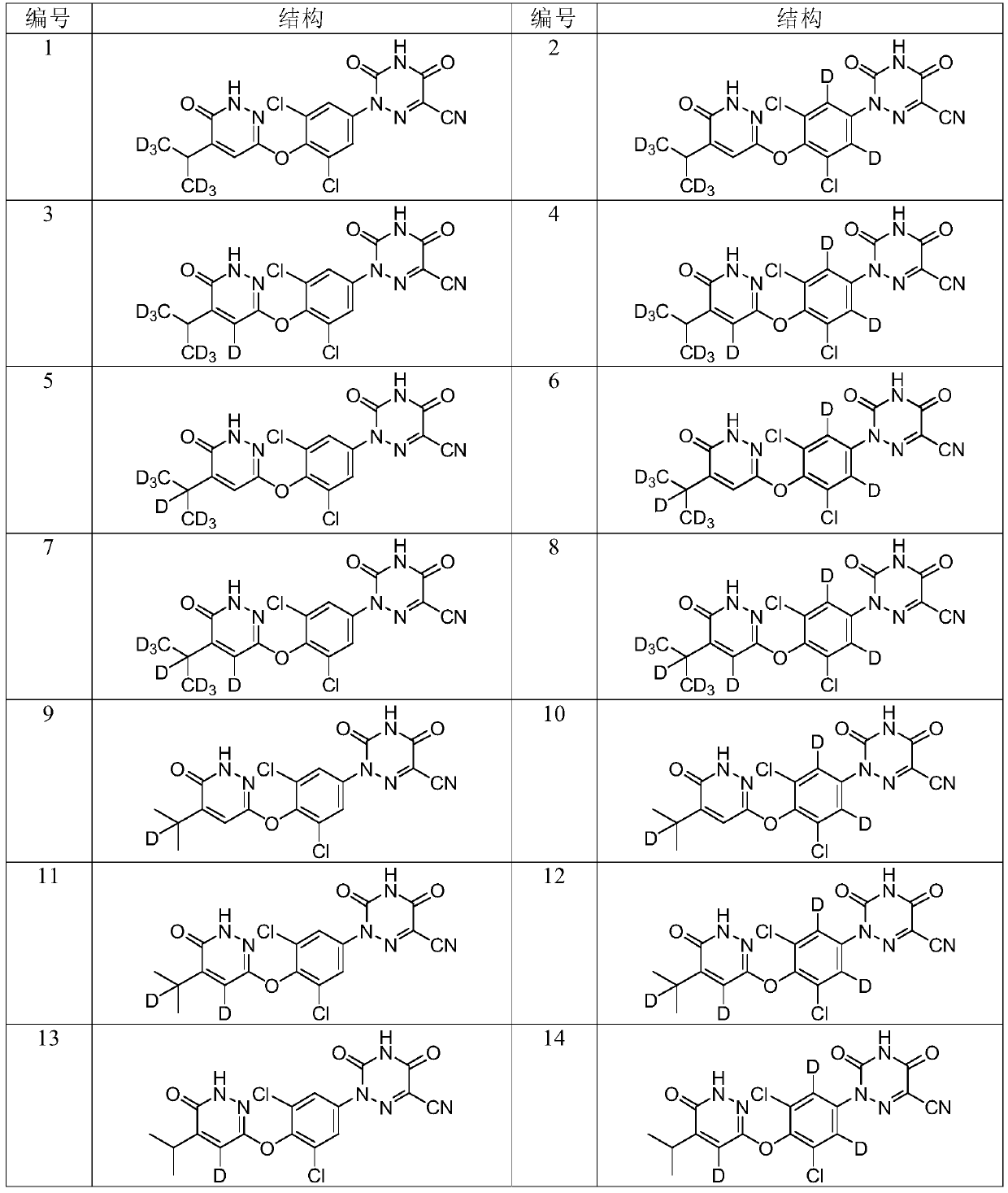
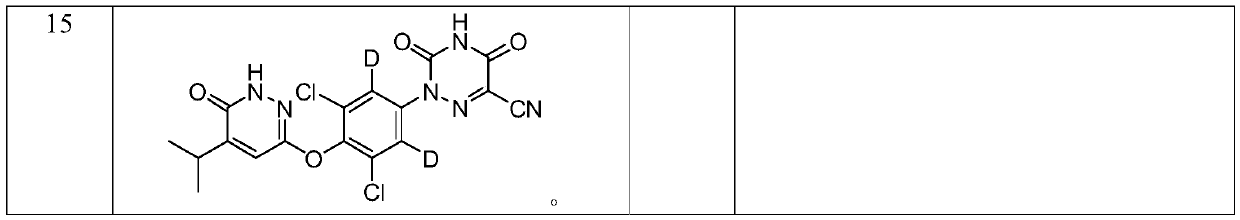
Deuterated pyridazinone and derivatives and pharmaceutical compositions thereof
A pyridazinone, deuterated technology, applied in the field of deuterated pyridazinone and its derivatives and pharmaceutical compositions, can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1 2-(3,5-dichloro-4-((6-oxo-5-(propan-2-yl-1,1,1,3,3,3-hexadeuterio)-1,6 Preparation of -dihydropyridazin-3-yl)oxy)phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile
[0140]
[0141] The first step: the preparation of 3,6-dichloro-4-(propan-2-yl-1,1,1,3,3,3-hexadeuterio)pyridazine
[0142] 2-(Trideuteriomethyl)-3,3,3-trideuteropropionic acid (1.4 g, 0.0151 mol) was added to 3,6-dichloropyridazine (2.25 g, 0.015 mol) at room temperature Acetonitrile (3.5mL), sulfolane (10.7mL) and water (24.5mL) mixed solution, followed by adding silver nitrate (1.3g, 0.mol). The reaction mixture was heated to 55°C, a solution of concentrated sulfuric acid (2.4mL) in water (7.5mL) was added in one portion, and a solution of ammonium persulfate (5.2g, 0.022mol) in water (7.5mL) was added dropwise within 35 minutes. Reaction The mixture was reacted at 70°C for 20 minutes and then cooled to room temperature and stirred at room temperature for 24 hours. The res...
Embodiment 2
[0157] Example 2 2-(3,5-dichloro-4-((6-oxo-5-(propan-2-yl-heptadeuterio)-1,6-dihydropyridazin-3-yl)oxy) Preparation of phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile
[0158]
[0159] LC-MS: m / z 442 (M+H) + . 1 H NMR (400MHz, DMSO-d 6 )δ13.25(brs,1H),12.23(s,1H),7.79(s,2H),7.44(s,1H).
Embodiment 3
[0160] Example 3 2-(3,5-dichloro-4-((6-oxo-5-(propan-2-yl-2-deutero)-1,6-dihydropyridazin-3-yl)oxy )Phenyl)-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile
[0161]
[0162] LC-MS: m / z 436 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com