Application of ethanone compound in preparation of medicine for treating tumors
An ethyl ketone and drug technology, which is applied in the application field of ethyl ketone compounds in the preparation of tumor drugs, and achieves the effect of promoting apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
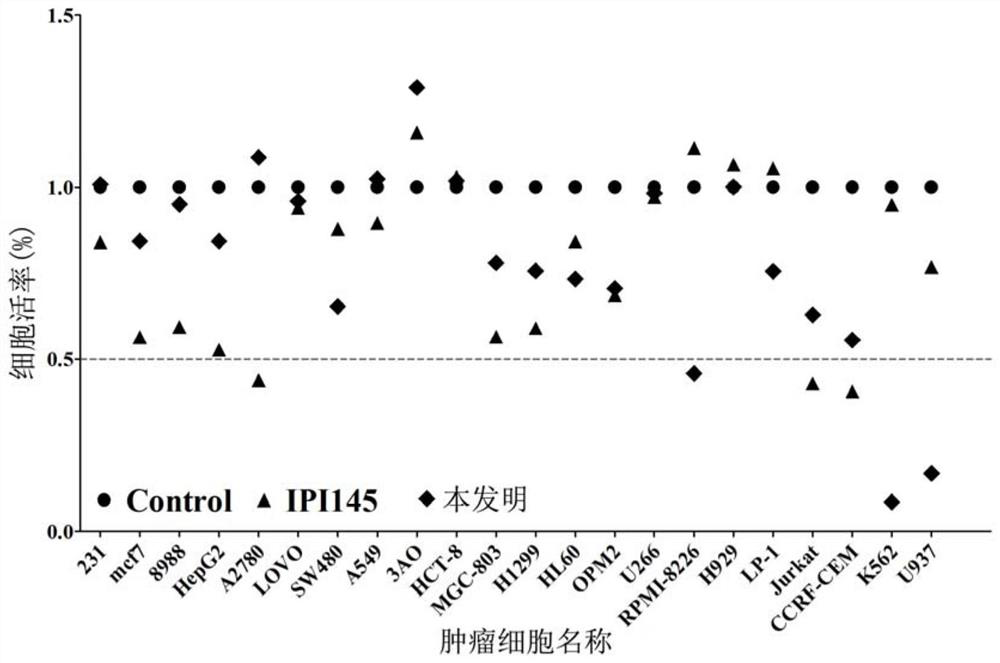
[0028] Embodiment 1 (in vitro multiple tumor cell proliferation inhibition verification compound inhibits tumor cell proliferation)
[0029] The MTT assay was used to detect the inhibitory effect of the compound on the proliferation of various tumor cells.
[0030] A selective PI3Kδ / γ inhibitor IPI-145 was used as a positive control.
[0031] Blank group: use a 96-well plate and set up 3 parallel experimental groups. A variety of tumor cells (including myeloma, leukemia, lymphoma, pancreatic cancer, gastric cancer, breast cancer, ovarian cancer, and lung cancer cell lines) were spread on a 96-well plate at a density of 7,500 cells per well. After incubation for 72 hours, add 10 μL MTT was incubated for 4 hours, 100 μL MTT Buffer was added to dissolve the cell crystals, and the wavelength was detected at 560 nm absorbance.
[0032] Experimental group: 3 groups of parallel experiments were set up using a 96-well plate. 1-(5-(3-((4-chlorobenzyl)amino)-2-hydroxypropoxy)-2-methy...
Embodiment 2
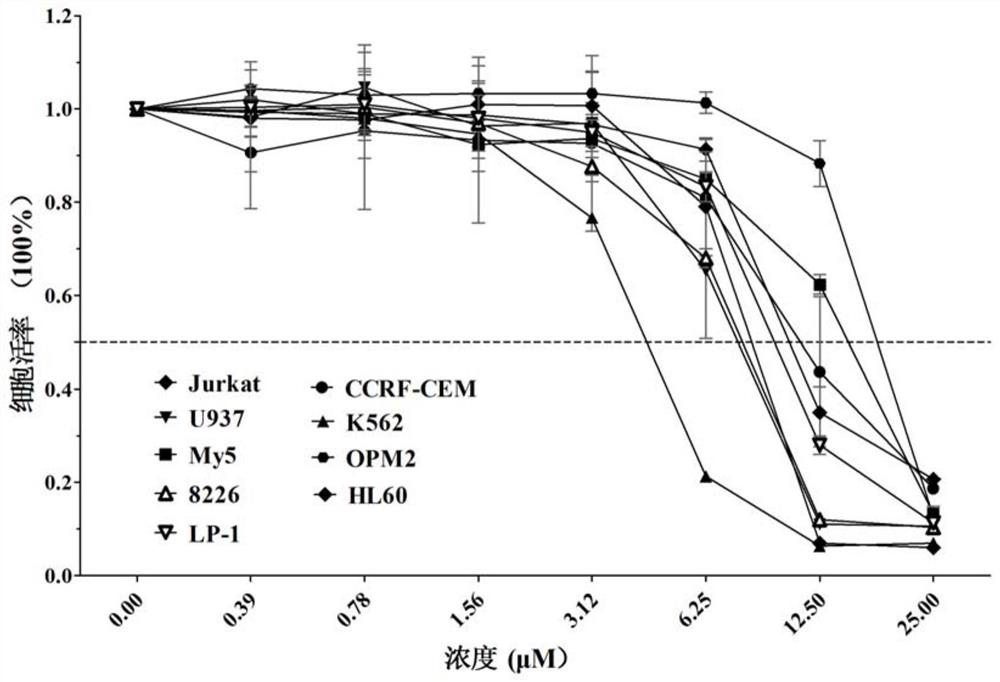
[0035] Example 2 (A variety of hematological tumor cells detect the inhibitory effect of active compounds on cell proliferation)
[0036] As can be seen from Example 1, 1-(5-(3-((4-chlorobenzyl) amino)-2-hydroxypropoxy)-2-methyl-1-(p-tolyl)-1 hydrogen -Indol-3-yl)ethanone has more obvious inhibition on hematological tumors, because in such tumor cells, PI3Kγ often presents a disordered high expression state, so the following examples select myeloma cells, lymphoma cells and leukemia cells Cells and other blood tumor cells were further tested and verified, and the MTT assay was used to detect different concentrations of the compound 1-(5-(3-((4-chlorobenzyl)amino)-2-hydroxypropoxy)-2-methanol Base-1-(p-tolyl)-1hydro-indol-3-yl)ethanone inhibits the proliferation of tumor cells.
[0037] Blank group: use a 96-well plate and set up 3 parallel experimental groups. A variety of myeloma cells OPM2, LP-1, RPMI-8226, lymphoma cells U937 and leukemia cells HL-60, Jurkat, CCRF-CEM, K5...
Embodiment 3
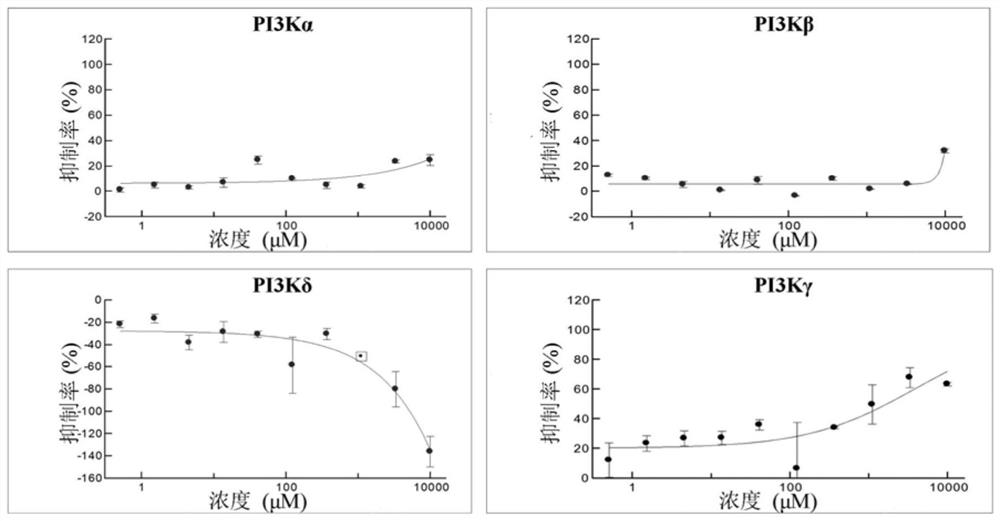
[0041] Embodiment 3 (in vitro enzyme assay detects PI3K inhibition rate)
[0042] Detection of compound 1-(5-(3-((4-chlorobenzyl)amino)-2-hydroxypropoxy)-2-methyl-1-(p-tolyl)-1 hydrogen using ADP-Glo kit Inhibitory activity of -indol-3-yl)ethanone on PI3Kα, PI3Kβ, PI3Kδ, PI3Kγ.
[0043] The broad-spectrum PI3K inhibitor PI-103 was used as a positive control.
[0044] PI3Kγ blank group: use a 384-well plate to set up 3 parallel experimental groups. Prepare 5 μL of PI3K reaction system, each well contains 2.5 μL of PI3Kγ protein, add 2.5 μL of reaction substrate PIP2 and ATP to start the reaction, incubate at room temperature for 1 hour, use 5 μL of MgCl 2 The reagent terminated the reaction, and after incubation for 2 hours, 10 μL of ADP-Glo detection reagent was added, and after incubation for 45 minutes, the fluorescence value of the reaction solution was detected by a microplate reader.
[0045] Use the same operation to prepare PI3Kα, PI3Kβ, PI3Kδ blank groups
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com