A kind of fluorescein conjugate and its synthesis method and application
A synthetic method, fluorescein technology, applied in the direction of chemical instruments and methods, methods based on microorganisms, biochemical equipment and methods, etc., can solve problems such as complex operations, unsatisfactory effects, and insignificant specificity, and achieve safety high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis of embodiment 1 fluorescein conjugate I-1
[0056] Synthesis of fluorescein conjugate I-1:
[0057]
[0058] Compound 2 (4.8mg, 12.3μmol, 1eq) and 1 (5.6mg, 12.3μmol, 1eq) were dissolved in water (1.5mL)), and triethylamine (8.5μL, 61.5μmol, 5eq) was added at room temperature, and stirred for 10 hours, Purification by HPLC gave 3 (2.3 mg, 2.71 μmol, 22%) as a yellow solid.
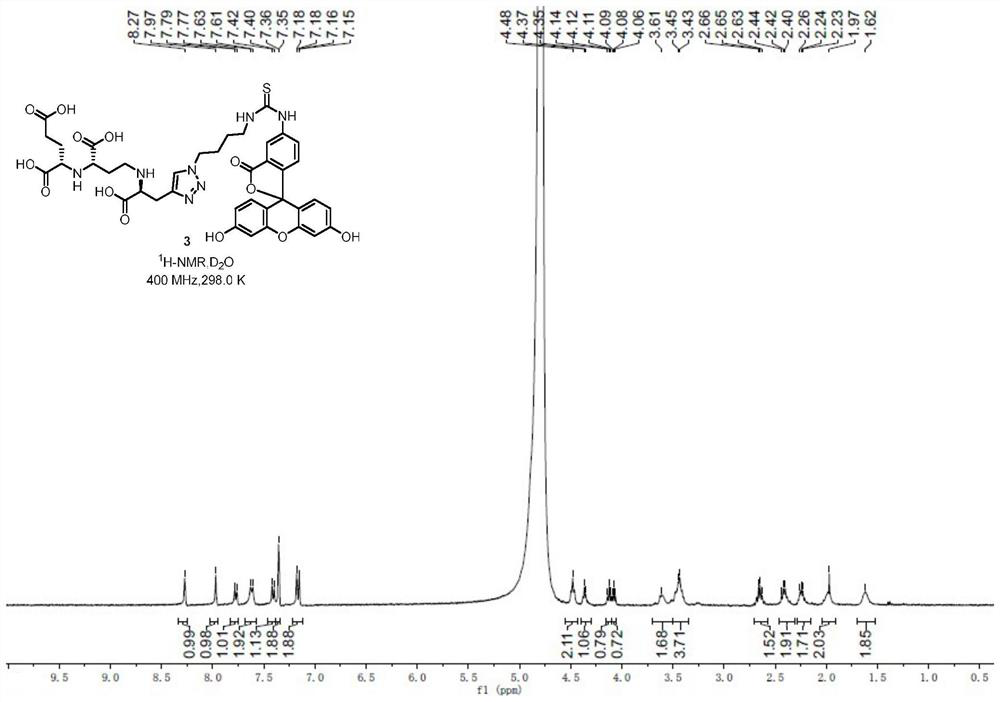
[0059] 1 H NMR (400MHz,D 2 O)δ8.27(s,1H),7.97(s,1H),7.78(d,J=8.4Hz,1H),7.62(d,J=9.8Hz,2H),7.41(d,J=8.1Hz ,1H),7.35(d,J=2.0Hz,2H),7.17(dd,J=9.2,2.0Hz,2H),4.48(t,J=5.6Hz,2H),4.36(d,J=5.7Hz ,1H),4.12(t,J=6.4Hz,1H),4.08(t,J=6.4Hz,1H),3.64–3.56(m,2H),3.50–3.38(m,4H),2.72–2.57( m,2H),2.48–2.33(m,2H),2.29–2.16(m,2H),2.03–1.93(m,2H),1.65–1.56(m,2H)( Figure 9 ); 13 C NMR (239MHz,D 2 O / CD 3 CN)δ180.8,176.2,171.3,170.8,170.2,168.7,166.0,156.7,141.0,140.7,131.8,129.8,129.6,128.5,124.9,117.3,114.4,102.9,100.0,62.8,60.3 44.0, 29.9, 27.1, 26.6, 25.5, 25.2, 25.1 ( Figure 10 ); IR(neat)ν m...
Embodiment 2
[0060] Synthesis of Example 2 Fluorescein Conjugate I-2
[0061] Synthesis of Fluorescein Conjugate I-2:
[0062]
[0063] Compound 1 (3.5mg, 6.4μmol, 1eq) and sodium bicarbonate (2.7mg, 32μmol, 5eq) were dissolved in water (1mL), and acetonitrile (1mL) and compound 4 (6.0mg, 7.7μmol, 1.2eq) were added at room temperature, After stirring for 2 hours, the green solid 5 (3.8 mg, 3.0 μmol, 47%) was obtained by gel column purification.
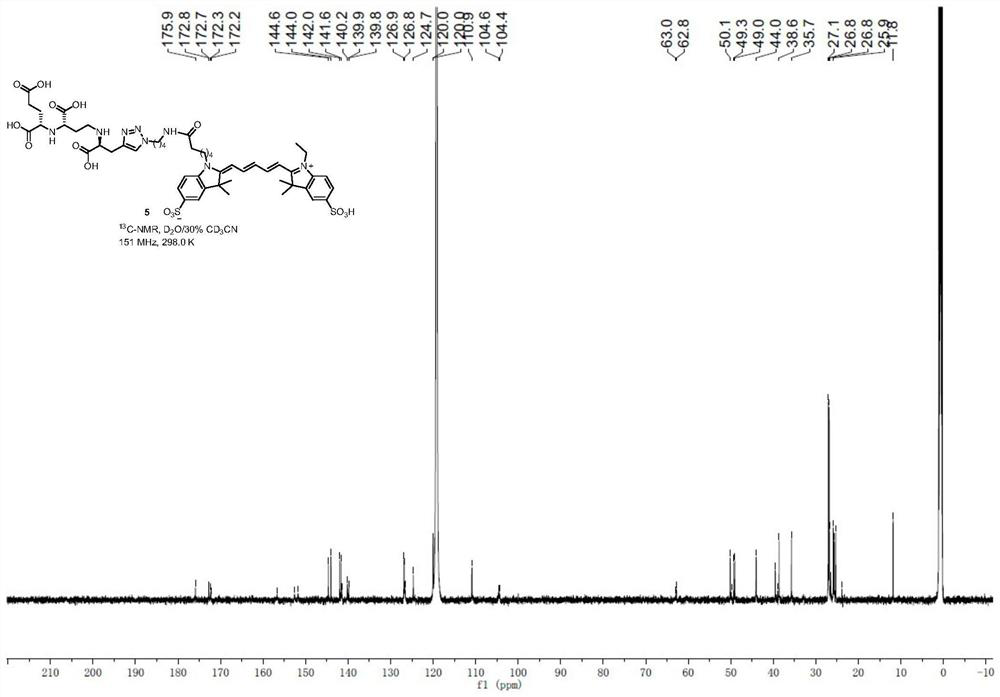
[0064] 1 H NMR (600MHz,D 2 O / 30%CD 3 CN)δ8.66(s,1H),8.21–8.13(m,2H),8.11–8.04(m,5H),7.88–7.77(s,1H),7.55(d,J=30.5Hz,2H), 6.89–6.73(m,2H),6.53(dd,J=37.9,12.1Hz,2H),4.60(br.s,2H),4.33(br.s,2H),4.27(br.s,2H), 4.18(br.s,1H),3.96–3.82(m,2H),3.54(br.s,4H),3.33(br.s,2H),2.72(br.s,2H),2.41(br.s ,2H),2.08(br.s,2H),2.03(br.s,3H),1.93(s,12H),1.84(br.s,3H),1.67–1.50(m,9H)( Figure 11 ); 13 C NMR (151MHz,D 2 O / 30%CD 3 CN)δ175.9,172.8,172.7,172.3,172.2,156.7,152.6,151.8,144.6,144.0,142.0,141.6,141.5,140.2,139.9,139.8,126.9,126.8,126.7,1009.6,11,12 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com