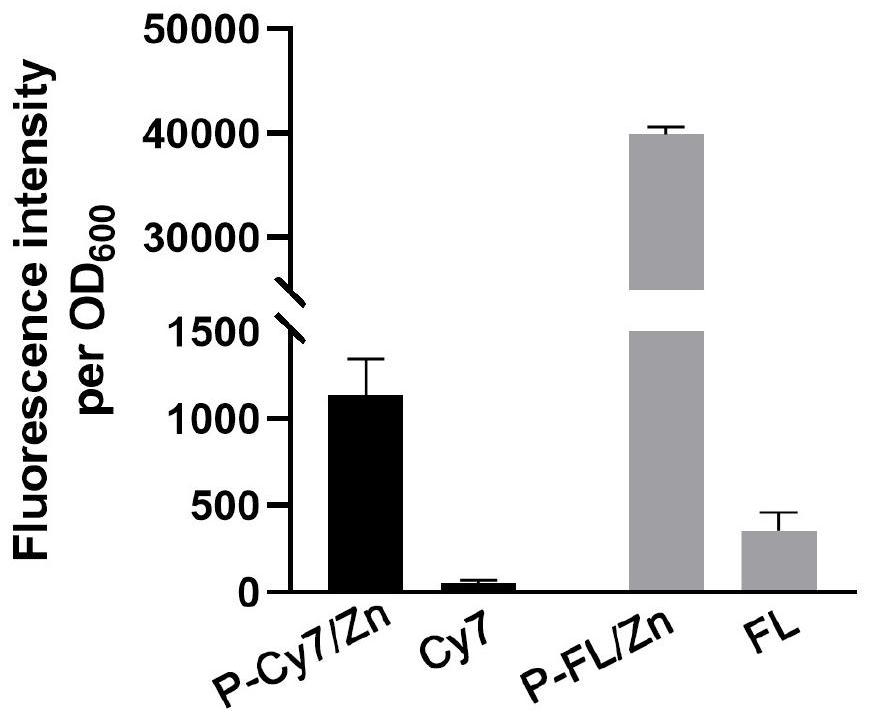
Fluorescein conjugate as well as synthesis method and application thereof
A synthetic method, fluorescein technology, applied in chemical instruments and methods, microorganism-based methods, biochemical equipment and methods, etc., can solve problems such as unsatisfactory effects, insignificant specificity, complicated operations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis of embodiment 1 fluorescein conjugate I-1
[0056] Synthesis of fluorescein conjugate I-1:
[0057]
[0058] Compound 2 (4.8mg, 12.3μmol, 1eq) and 1 (5.6mg, 12.3μmol, 1eq) were dissolved in water (1.5mL)), and triethylamine (8.5μL, 61.5μmol, 5eq) was added at room temperature, and stirred for 10 hours, Purification by HPLC gave 3 (2.3 mg, 2.71 μmol, 22%) as a yellow solid.
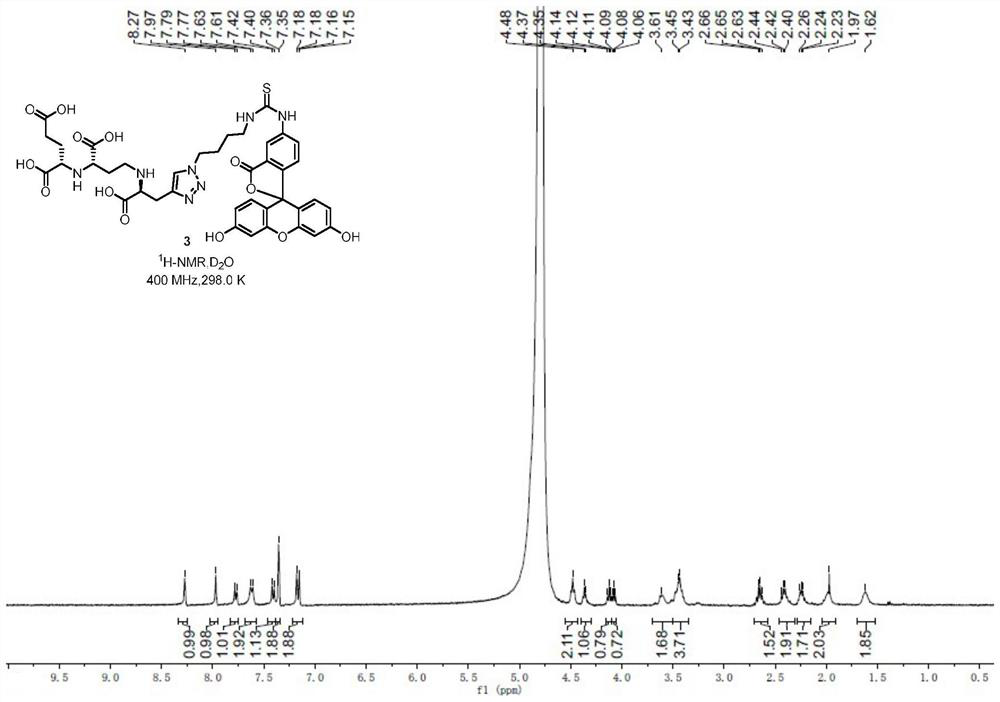
[0059] 1 H NMR (400MHz,D 2 O)δ8.27(s,1H),7.97(s,1H),7.78(d,J=8.4Hz,1H),7.62(d,J=9.8Hz,2H),7.41(d,J=8.1Hz ,1H),7.35(d,J=2.0Hz,2H),7.17(dd,J=9.2,2.0Hz,2H),4.48(t,J=5.6Hz,2H),4.36(d,J=5.7Hz ,1H),4.12(t,J=6.4Hz,1H),4.08(t,J=6.4Hz,1H),3.64–3.56(m,2H),3.50–3.38(m,4H),2.72–2.57( m,2H),2.48–2.33(m,2H),2.29–2.16(m,2H),2.03–1.93(m,2H),1.65–1.56(m,2H)( Figure 9 ); 13 C NMR (239MHz,D 2 O / CD 3 CN)δ180.8,176.2,171.3,170.8,170.2,168.7,166.0,156.7,141.0,140.7,131.8,129.8,129.6,128.5,124.9,117.3,114.4,102.9,100.0,62.8,60.3 44.0, 29.9, 27.1, 26.6, 25.5, 25.2, 25.1 ( Figure 10 ); IR(neat)ν m...
Embodiment 2
[0060] Synthesis of Example 2 Fluorescein Conjugate I-2
[0061] Synthesis of Fluorescein Conjugate I-2:
[0062]
[0063] Compound 1 (3.5mg, 6.4μmol, 1eq) and sodium bicarbonate (2.7mg, 32μmol, 5eq) were dissolved in water (1mL), and acetonitrile (1mL) and compound 4 (6.0mg, 7.7μmol, 1.2eq) were added at room temperature, After stirring for 2 hours, the green solid 5 (3.8 mg, 3.0 μmol, 47%) was obtained by gel column purification.
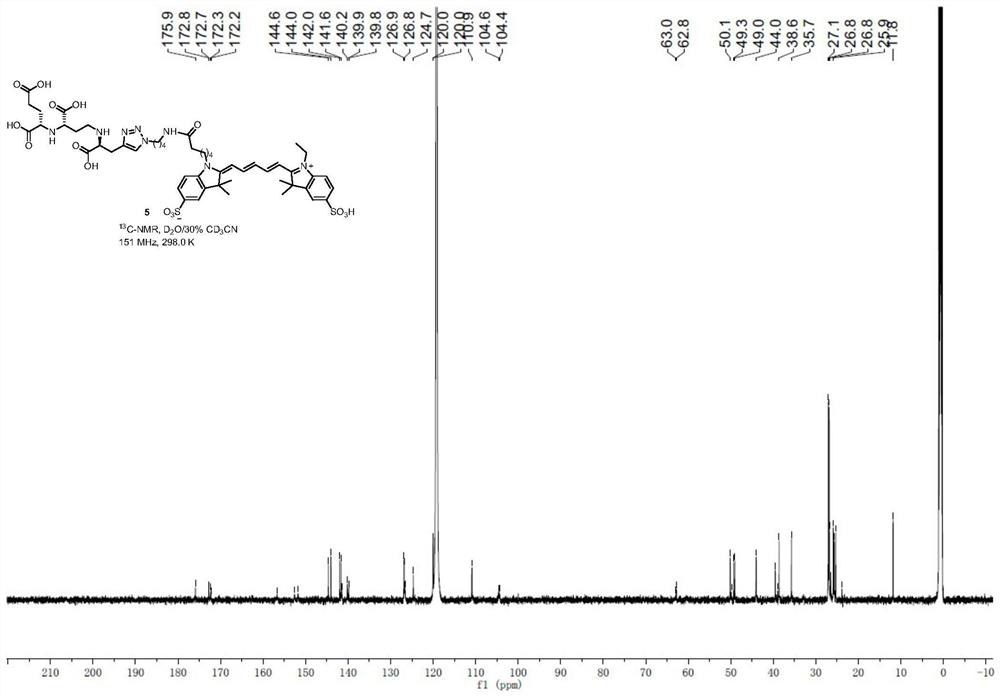
[0064] 1 H NMR (600MHz,D 2 O / 30%CD 3 CN)δ8.66(s,1H),8.21–8.13(m,2H),8.11–8.04(m,5H),7.88–7.77(s,1H),7.55(d,J=30.5Hz,2H), 6.89–6.73(m,2H),6.53(dd,J=37.9,12.1Hz,2H),4.60(br.s,2H),4.33(br.s,2H),4.27(br.s,2H), 4.18(br.s,1H),3.96–3.82(m,2H),3.54(br.s,4H),3.33(br.s,2H),2.72(br.s,2H),2.41(br.s ,2H),2.08(br.s,2H),2.03(br.s,3H),1.93(s,12H),1.84(br.s,3H),1.67–1.50(m,9H)( Figure 11 ); 13 C NMR (151MHz, D 2 O / 30%CD 3 CN)δ175.9,172.8,172.7,172.3,172.2,156.7,152.6,151.8,144.6,144.0,142.0,141.6,141.5,140.2,139.9,139.8,126.9,126.8,126.7,1009.6,11,12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com