Lactamase and its application and method for enzymatic resolution and preparation of (1r, 4s)-venslide
A technology of lactamase and vince lactone, which is applied in the field of bioengineering, can solve the problems of low tolerance and activity, lower production efficiency, and low substrate concentration, so as to reduce production costs, improve production efficiency, The effect of high substrate tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
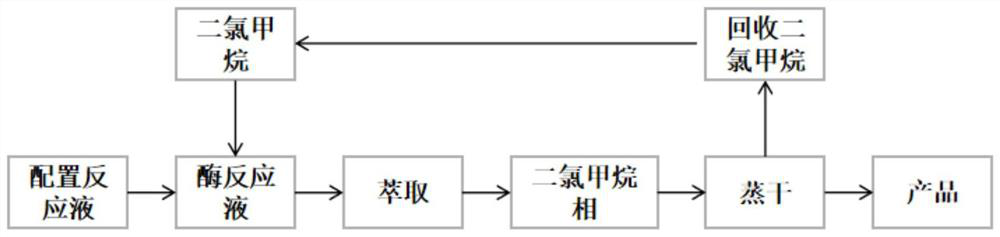
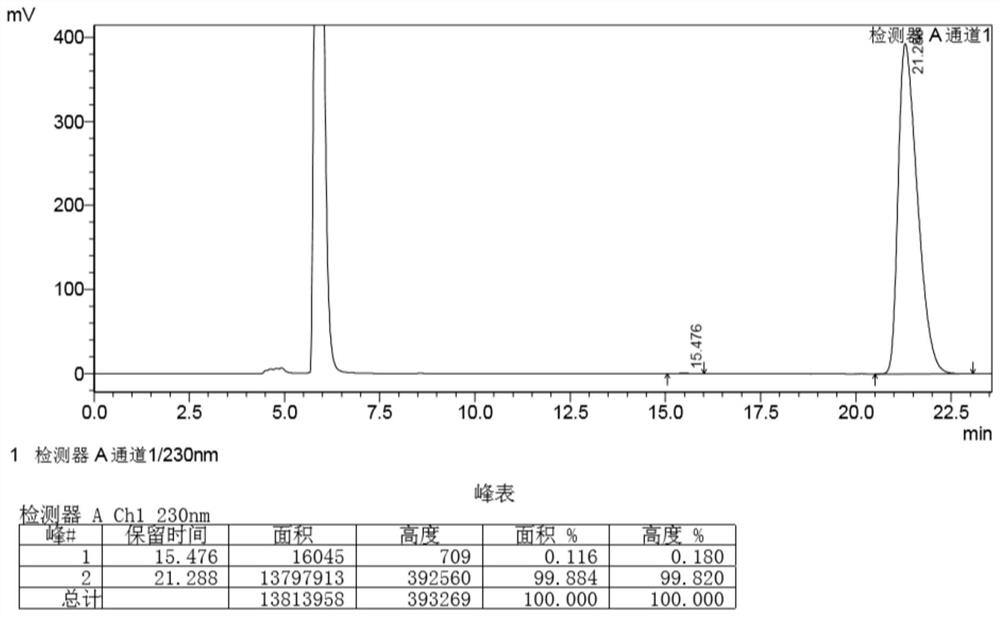
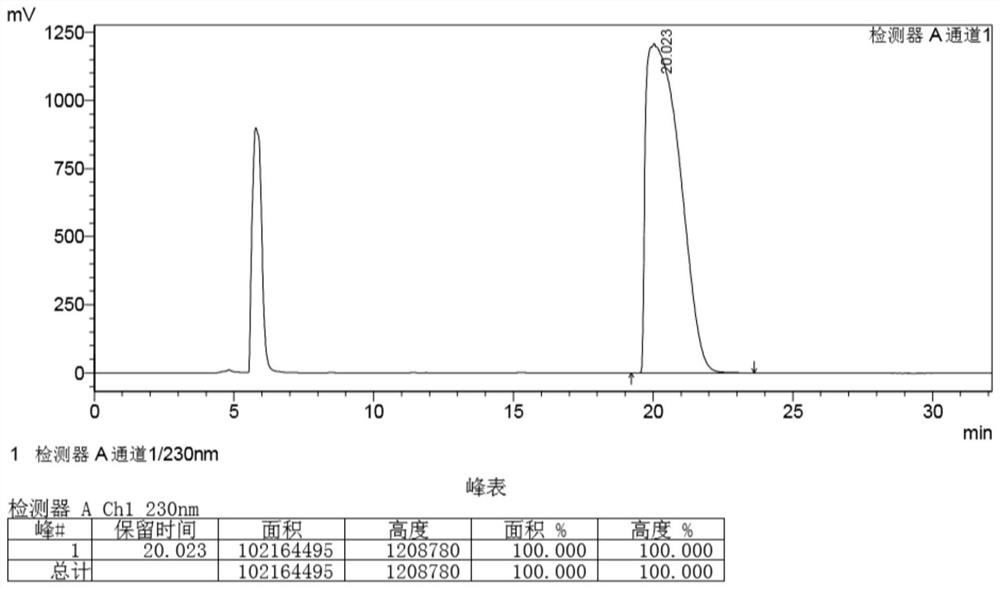
[0041] The method for the enzymatic resolution and preparation of (1R,4S)-venslide provided in this example specifically includes the following steps: dissolving 500 g of the substrate venslide with 1 L phosphate buffer solution with a concentration of 10 mM, and then adding lactam Enzyme 3g, start stirring, react at 25°C, take samples in the middle and control in the middle, after the reaction ≥ 99% (see figure 2), start post-processing, add 4% dichloromethane to the reaction system, stir for 10 minutes, then concentrate to 3 / 5 volume, use 2.5 times volume of dichloromethane to extract 3 times (1 times, 1 times, 0.5 times ), the concentrated dichloromethane phase after the extraction was completed, the solvent was recovered, and evaporated to dryness to obtain the target product (1R, 4S)-2-azabicyclo[2.2.1]hept-5-en-3-one 135.9g, yield 48.8%, chiral purity 100% (see image 3 ).
[0042] in, figure 2 and image 3 They are the detection result diagrams of the high perform...
Embodiment 2
[0054] The method for the enzymatic resolution and preparation of (1R,4S)-venslide provided in this example specifically includes the following steps: dissolving 500 g of the substrate venslide with 1 L phosphate buffer solution with a concentration of 10 mM, and then adding lactam Enzyme 3g, start stirring, react at 15°C, take samples in the middle and control in the middle, after the reaction is ≥99%, start post-processing, add 4% dichloromethane to the reaction system, stir for 10 minutes, then concentrate to 3 / 5 volume, use 2.5 times the volume of dichloromethane was extracted 3 times (1 times, 1 times, 0.5 times), after the extraction, the dichloromethane phase was concentrated, the solvent was recovered, and the target product (1R,4S)-2-azabicyclo was obtained after evaporation to dryness [2.2.1] 135.9 g of hept-5-en-3-one.
Embodiment 3
[0056] The method for the enzymatic resolution and preparation of (1R,4S)-venslide provided in this example specifically includes the following steps: dissolving 500 g of the substrate venslide with 1 L phosphate buffer solution with a concentration of 10 mM, and then adding lactam Enzyme 3g, start stirring, react at 20°C, take samples in the middle and control in the middle, after the reaction is ≥99%, start post-processing, add 4% dichloromethane to the reaction system, stir for 10 minutes, then concentrate to 3 / 5 volume, use 2.5 times the volume of dichloromethane was extracted 3 times (1 times, 1 times, 0.5 times), after the extraction, the dichloromethane phase was concentrated, the solvent was recovered, and the target product (1R,4S)-2-azabicyclo was obtained after evaporation to dryness [2.2.1] 135.9 g of hept-5-en-3-one.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com