Compounds with antidiabetic activity in frangipani and preparation method thereof
An anti-diabetic and frangipani technology, applied in the field of compounds, can solve the problem of few reports on the active ingredients of frangipani
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
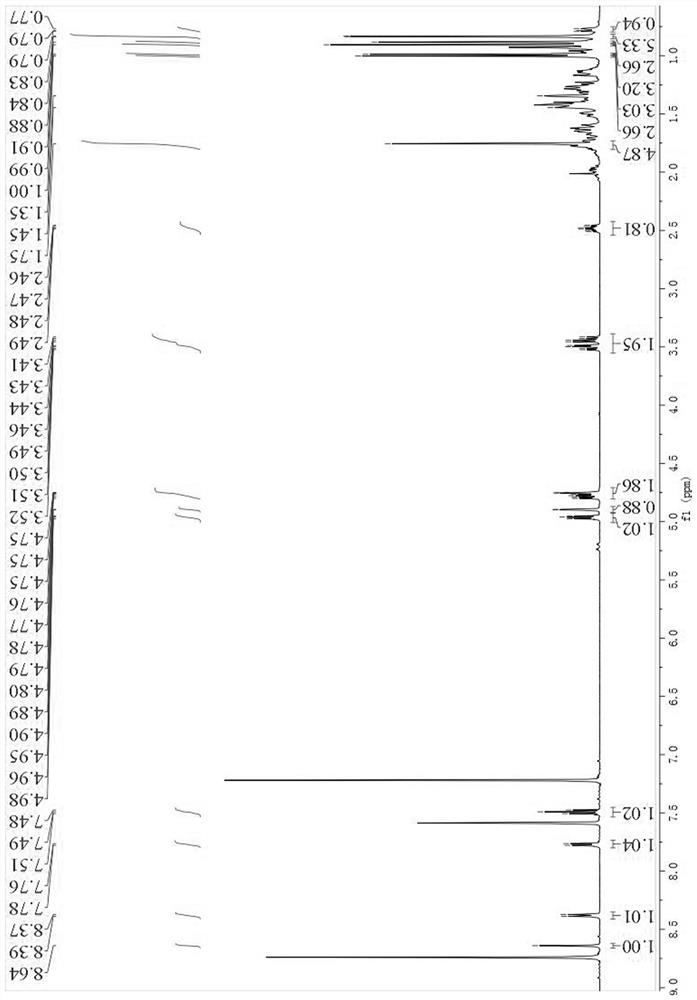
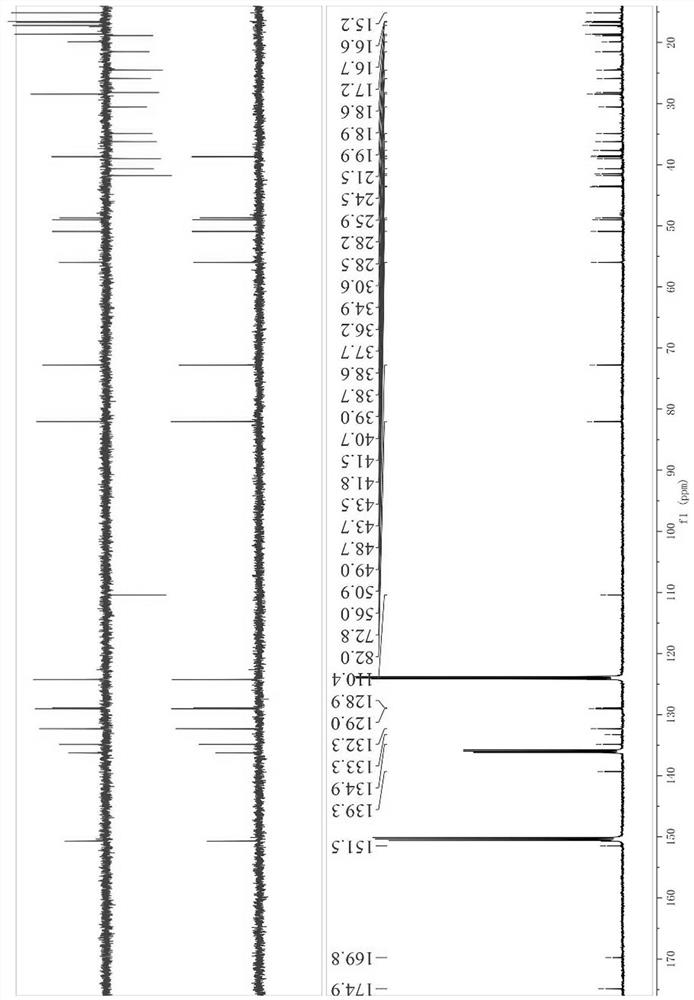
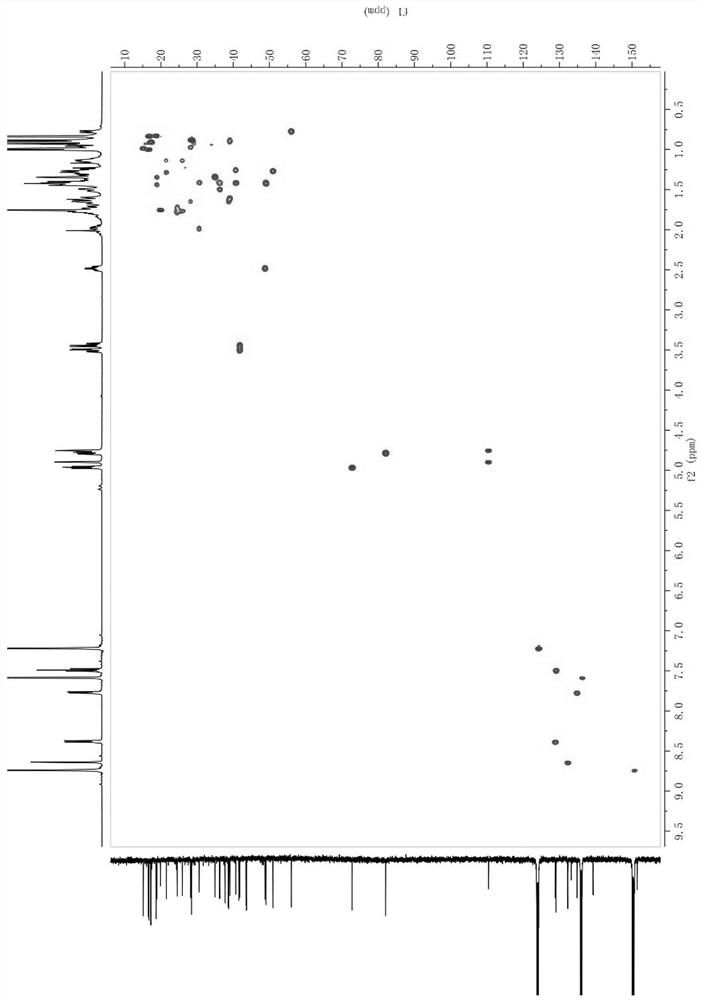
[0034] Structure identification of compound 1 by high-resolution mass spectrometry (HR-ESI-MS m / z : 617.4229 [M – H] ‒ , calcdfor C 40 H 57 O 5 ‒ 617.4211) can be inferred that its molecular formula is C 40 H 58 O 5 , with 12 degrees of unsaturation; +26.77 ( c 0.43, DMSO). IR (KBr) data showed OH ( υ max 3422 cm ‒1 ), COOH ( υ max 1715cm ‒1 ), meta-substituted benzene ring ( υ max 964, 882, 750 cm ‒1 ). UV (methanol) λ max (log ε ) 220 (3.46), 275 (1.89). 1 HNMR (500 MHz, pyridine- d 5 ) δ H : 0.90 (1H, m, H-1a), 1.62 (1H, m, H-1b), 1.77 (2H, m, H-2), 4.78 (1H, dd, J = 11.7, 4.8 Hz, H-3), 0.78 (1H, m, H-5), 1.35 (1H, m, H-6a), 1.45 (1H, m, H-6b), 1.35 (2H, m, H-7), 1.28 (1H, m, H-9), 1.29 (2H, m, H-11), 1.14 (2H, m, H-12), 1.62 (1H, m, H-13), 0.97 (1H, m, H-15a), 1.67 (1H, m, H-15b), 1.42 (1H, m, H-16a), 1.50 (1H, m, H-16b), 1.42 (1H, m, H -18), 2.48 (1H, td, J = 11.0, 5.8 Hz, H-19), 1.41 (2H, m, H-21), 1.26 (1H, m, H-22a) 1.40 (1H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com