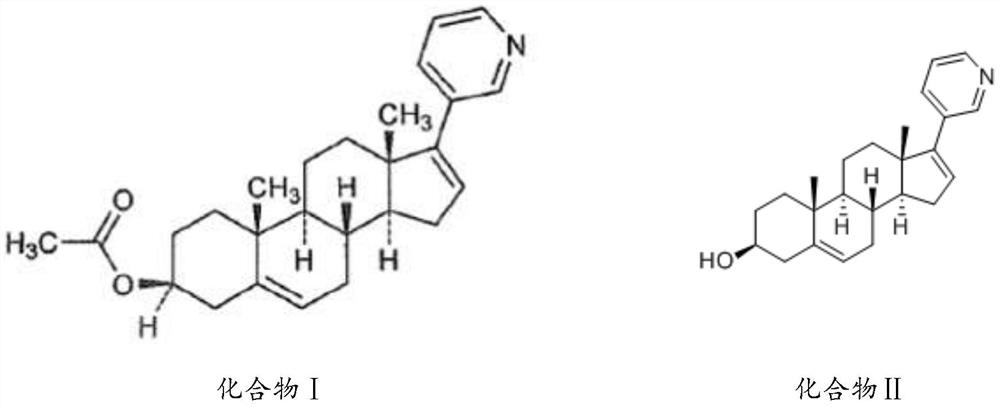
Application of composition containing cyclopenteno Cyclopenteno multi-hydrogen phenanthrene derivative in preparation of anti-tumor drug
A kind of technology of cyclopentene and derivatives, applied in the field of preparing medicines for anti-tumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 Oral solid preparation of a composition containing cyclopentene polyhydrophenanthrene derivatives and its preparation method
[0076] prescription
[0077]
[0078]
[0079]
[0080] Preparation
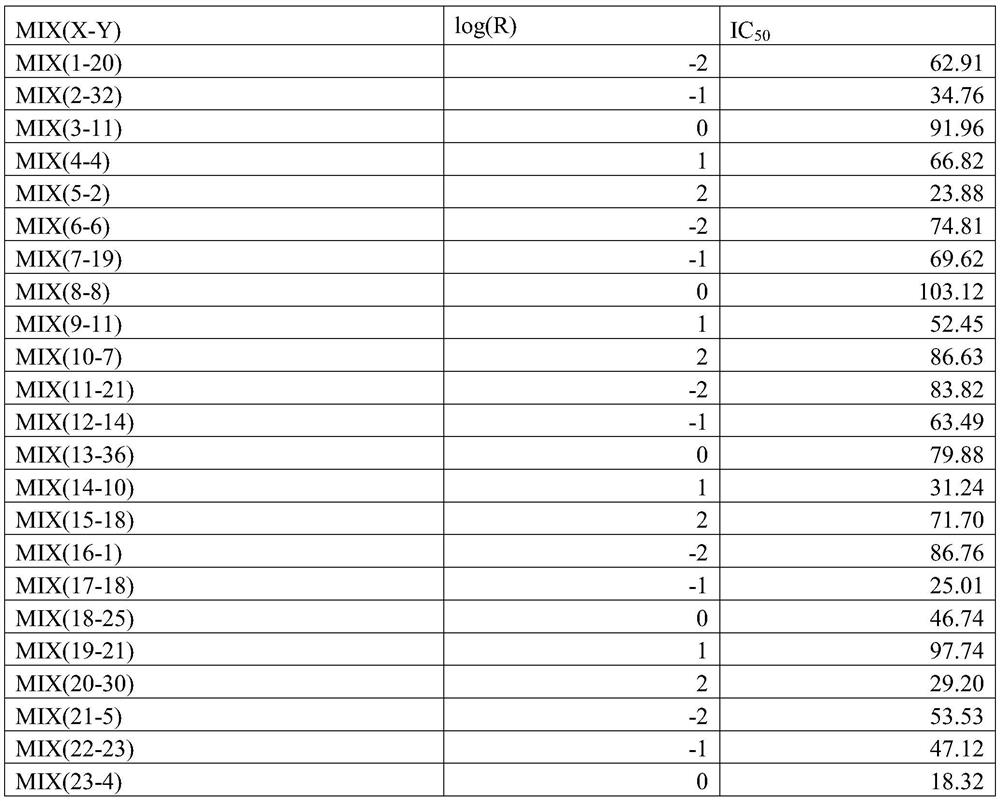
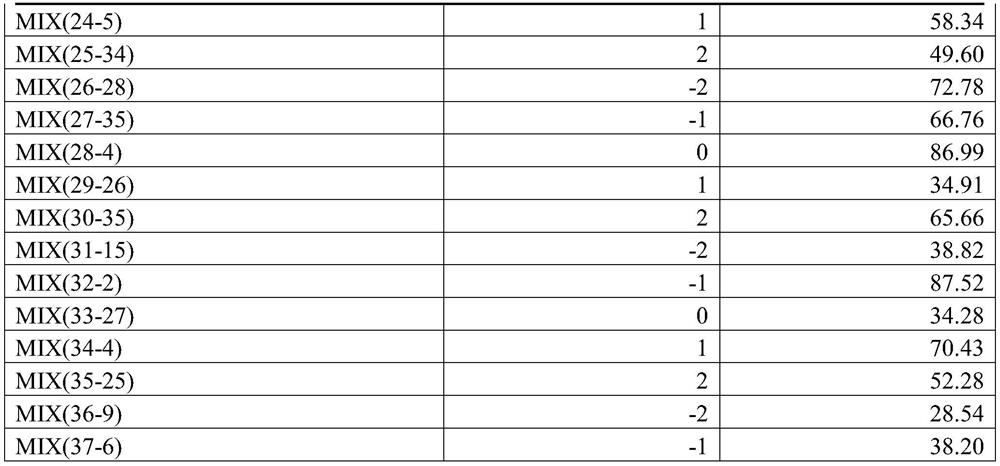
[0081] Take the prescribed amount of MIX (X-Y) and auxiliary materials, and pass through a 100-mesh sieve. Get MIX (X-Y), lactose, microcrystalline cellulose, crospovidone and starch and fully mix; get the prescription amount of hypromellose, and prepare a solution with a concentration of 10% based on hypromellose, Use lactic acid to adjust the pH to 3.0-4.0, add it to the above-mentioned mixture to make a soft material, granulate with a 16-mesh sieve, and dry at 80°C for 3-4 hours. Use a 16-mesh sieve to sieve the granules, add the prescribed amount of micropowder silica gel and magnesium stearate, mix well, fill the capsules, and each capsule weighs about 500mg;
[0082] Take the prescribed amount of MIX (X-Y) and auxiliary materials, and pass throu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com