Method for determining related substances of pharmaceutical preparation containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate
A technology of dextromethorphan hydrobromide and doxylamine succinate, applied in the field of medicine, can solve problems such as no simultaneous measurement, and achieve the effects of effective separation, saving detection time and detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] 1. Solution preparation
[0054] The diluent in the embodiment is a 0.01mol / L hydrochloric acid solution containing 20% by volume of methanol. The inventor has verified through experiments that the diluent has a good dissolving effect on acetaminophen, dextromethorphan hydrobromide, doxylamine succinate and related substances in the soft capsule.
[0055] (1) Preparation of test solution
[0056] Take the pharmaceutical preparation containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate, put it in a container, add diluent and heat, shake, dissolve and dilute to the mark, shake well, filter to obtain The test solution.
[0057] (2) Preparation of sensitivity solution
[0058] Take dextromethorphan impurity Ⅰ, dextromethorphan impurity Ⅱ, dextromethorphan impurity Ⅲ, dextromethorphan impurity Ⅳ, acetaminophen, doxylamine succinate and dextromethorphan hydrobromide, put them in a container, add dilute solution, ultrasonically dissolved, dilut...
experiment example 1
[0087] Establishment of detection method:
[0088] A method for detecting related substances in soft capsules containing acetaminophen, dextromethorphan hydrobromide and doxylamine succinate, which is carried out by high performance liquid chromatography. Include the following steps:
[0089] Preparation contains the soft capsule sample solution of paracetamol, dextromethorphan hydrobromide and doxylamine succinate, and described soft capsule sample solution comprises need testing solution, reference substance solution, system suitability solution and sensitivity solution.
[0090] Preparation of the test solution: Take the soft capsules containing paracetamol, dextromethorphan hydrobromide and doxylamine succinate, put them in a container, add diluent and heat, shake, dissolve and dilute to the mark , shake well, and filter to obtain the test solution. In the test solution, the concentration of acetaminophen was 9.1 mg / mL, the concentration of doxylamine succinate was 175 μ...
experiment example 2
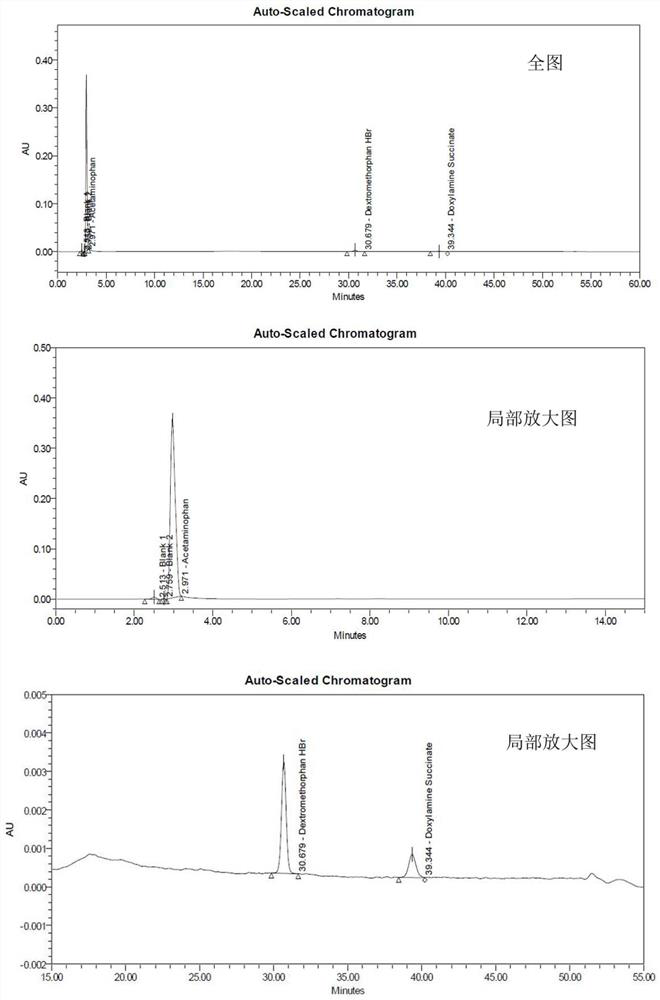
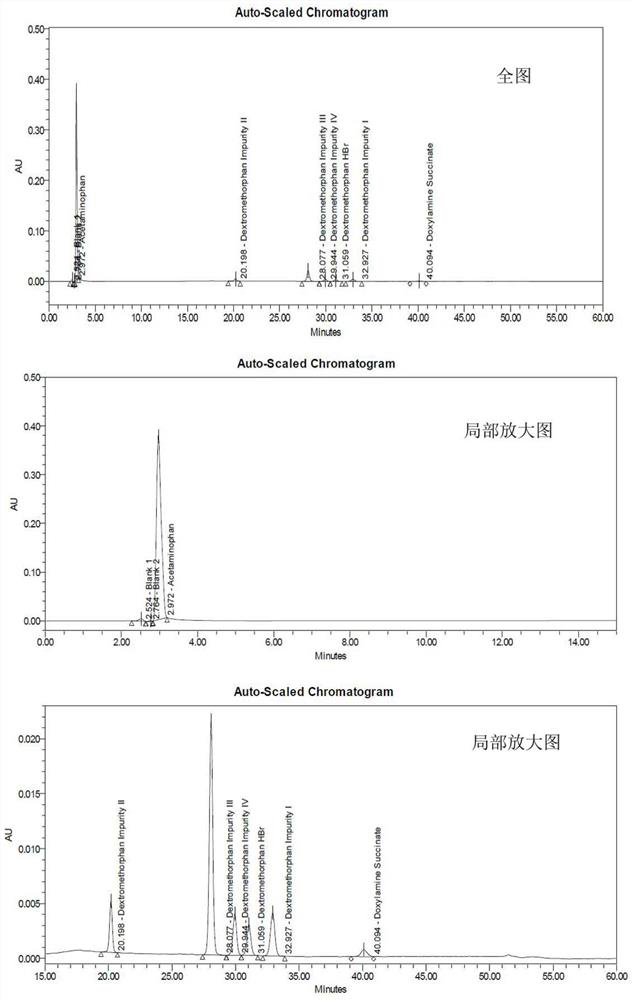
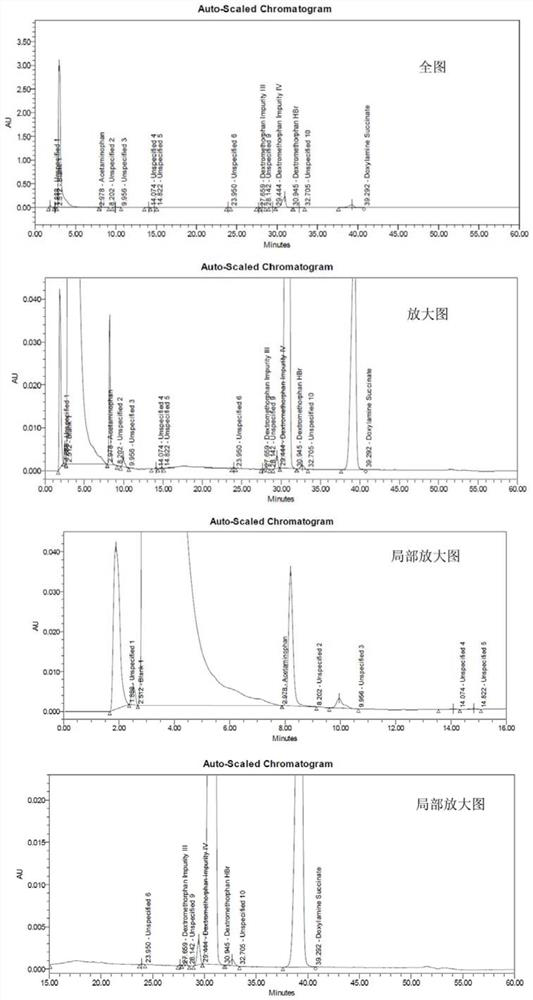
[0100] This experimental example has verified the feasibility of the chromatographic conditions used in the present invention. Specifically, adopt the chromatographic condition of above-mentioned experimental method to measure respectively reference substance solution, system adaptability solution and test solution, from Figure 1~3 It can be seen from Tables 2 to 3 that there is a good separation between the three main component peaks and their related substance peaks in the system adaptability solution collected by using the HPLC conditions of the present invention. There are no other interference peaks near the three main component peaks in the test solution collected by adopting the HPLC conditions of the present invention, and the separation degree is good.
[0101] Table 2 System adaptability test results
[0102]
[0103] Table 3 test result of test solution
[0104]
[0105]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com