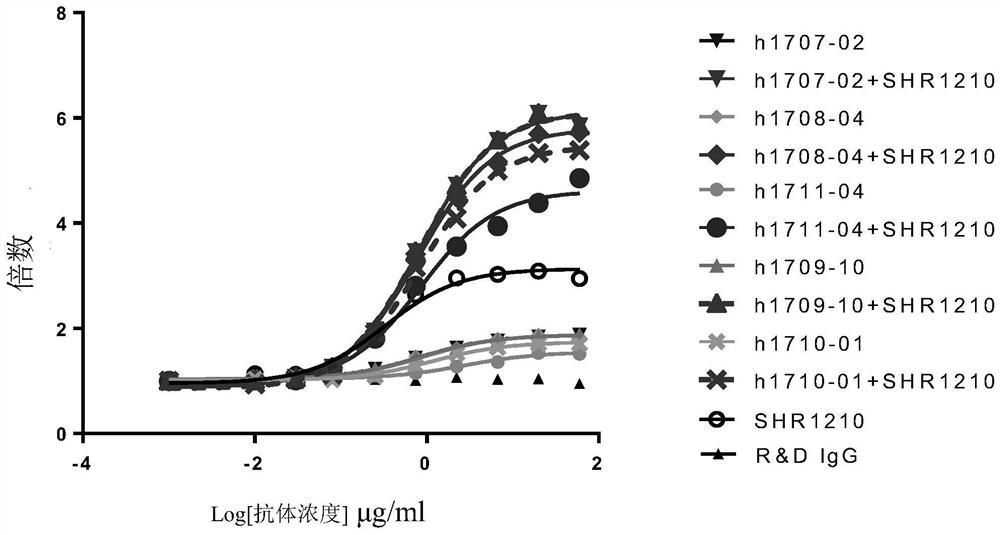
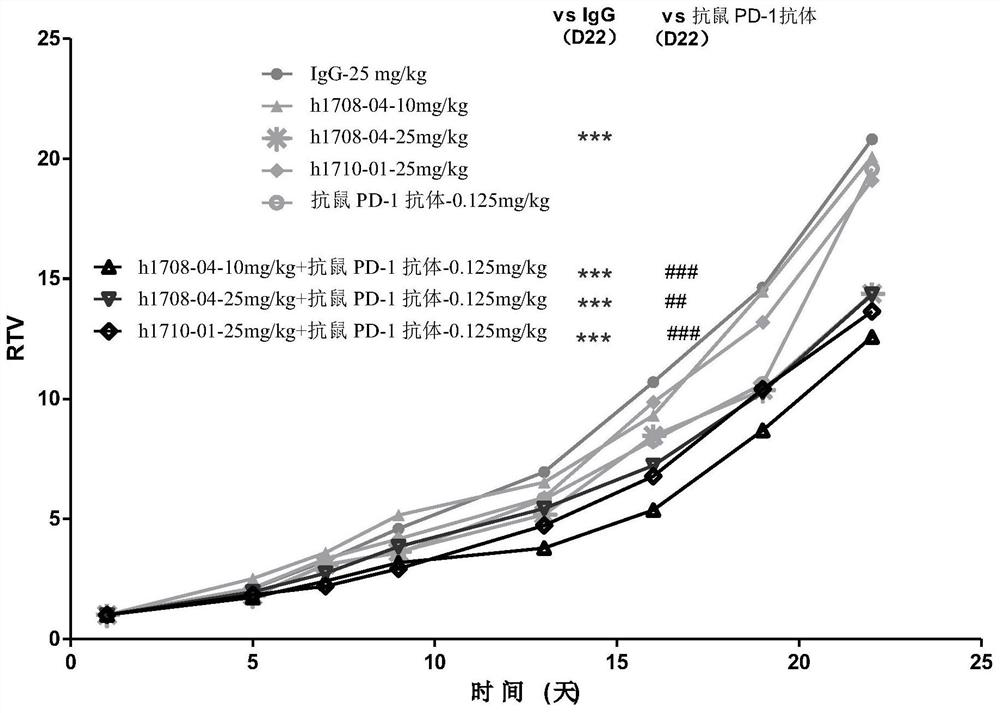
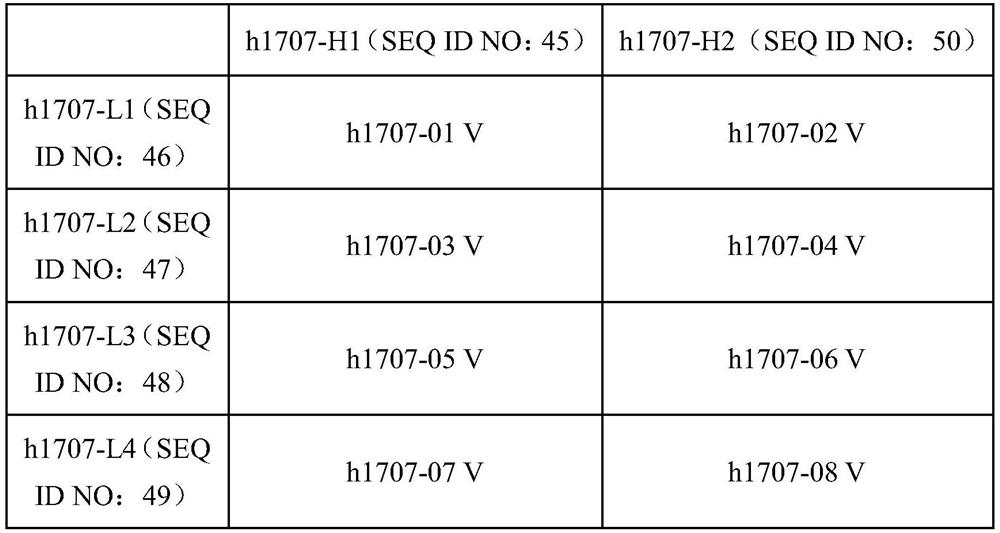
Method for treatment of disease by anti-TIGIT antibody in combination with PD-1 inhibitor and pharmaceutical combination
A PD-1 and inhibitor technology, applied in the direction of drug combination, antibody medical components, antibodies, etc., can solve the problem of disappearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Embodiment 1, the preparation of TIGIT antigen antibody
[0175] 1.1 Protein design and expression
[0176] Using human TIGIT protein (Uniprot number: Q495A1) as the template of TIGIT of the present disclosure, design the amino acid sequence of the antigen and detection protein of the present disclosure, optionally fuse different tags on the basis of TIGIT protein, and clone them respectively on the pHr vector ( Self-produced) or pXC-17.4 vector (LONZA), transiently expressed in 293 cells or stably expressed and purified in CHO cells, to obtain the protein encoding the disclosed antigen and detection. The following TIGIT antigens refer to human TIGIT unless otherwise specified.
[0177] Fusion protein of TIGIT extracellular region and mouse IgG2aFc fragment: TIGIT-mFc, for immunization and detection
[0178] MEFGLSWLFLVAILKGVQC MMTGTIETTGNISAEKGGSIILQCHLSSTTAQVTQVNWEQQDQLLAICNADLGWHISPSFKDRVAPGPGLGLTLQSLTVNDTGEYFCIYHTYPDGTYTGRIFLEVLESSVAEHGARFQIPEPRGPTIKPCPPCKCPAPNL...
Embodiment 2
[0197] Embodiment 2, preparation of anti-human TIGIT hybridoma monoclonal antibody
[0198] 2.1 Immunity
[0199] Anti-human TIGIT monoclonal antibody was produced by immunizing mice. SJL white mice used in the experiment, female, 6-8 weeks old (Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., animal production license number: SCXK (Beijing) 2012-0001). Breeding environment: SPF grade. After the mice were purchased, they were raised in a laboratory environment for 1 week, with a 12 / 12 hour light / dark cycle adjustment, a temperature of 20-25° C., and a humidity of 40-60%. The acclimatized mice were immunized according to the following scheme. The immunizing antigen is the extracellular region of human TIGIT with mFc (SEQ ID NO: 1).
[0200] Immunization regimen: with Gold Adjuvant (Sigma Cat No.T2684) and Thermo Alum (Thermo Cat No.77161) adjuvant cross-immunization. Antigen and adjuvant ( Gold Adjuvant) ratio of 1:1, antigen and adjuvant (Thermo Alu...
Embodiment 3
[0242] Example 3, Humanization of mouse-derived anti-human TIGIT antibody
[0243] By comparing the IMGT human antibody heavy and light chain variable region germline database and MOE software, the heavy chain and light chain variable region germline genes with high homology to the mouse antibody were selected as templates, and the mouse antibody The CDRs are respectively transplanted into corresponding human templates to form variable region sequences in the order of FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4. Back-mutation was carried out on the amino acids in the FR region as required to obtain a humanized anti-TIGIT monoclonal antibody. In the following exemplary specific examples, the determination of the amino acid residues in the CDR region is determined and annotated by the Kabat numbering system.
[0244] The light and heavy chain variable regions of the above murine antibodies are linked with the light and heavy chain constant regions of the human antibody to form a chimeric an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com