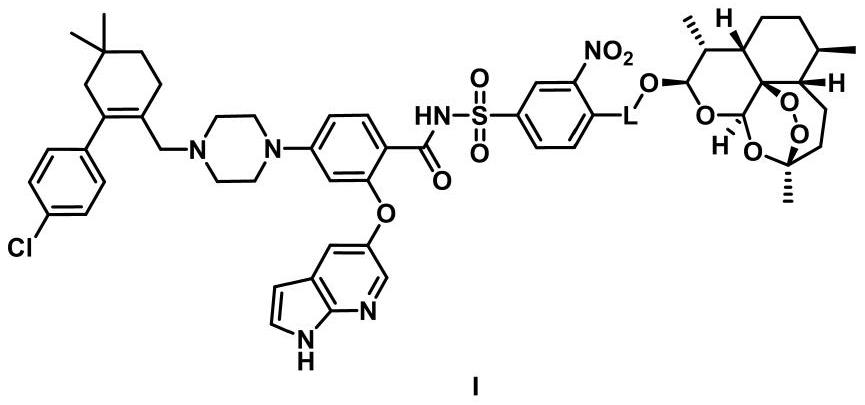
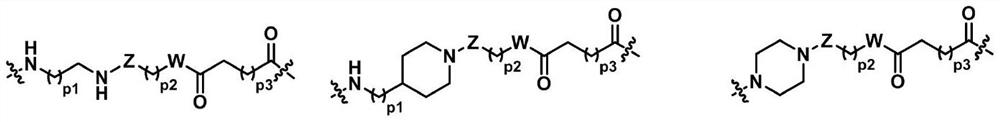
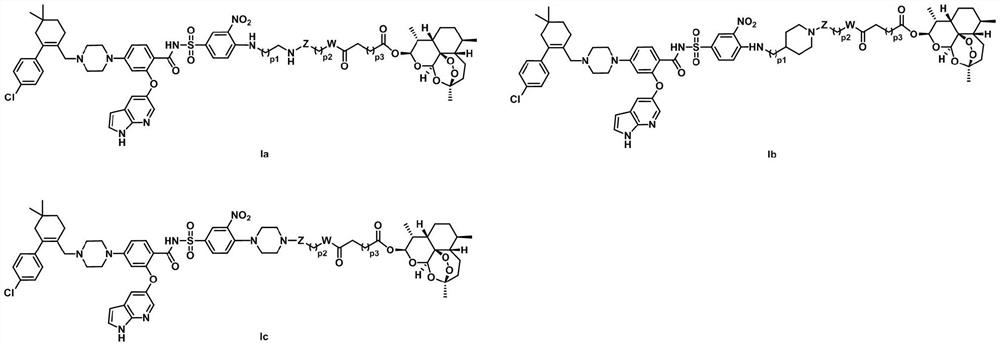
Venetoclax and dihydroartemisinin compound and preparation method and application thereof
A technology of dihydroartemisinin and compound, applied in the field of medicine, can solve the problem of narrow indication of venetola
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118]Example 1: 4-((2-((4-(N-(2-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-(( 4'-Chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl)benzene Formyl)sulfonamido)-2-nitrophenyl)amino)ethyl)amino)-4-oxobutanoic acid-(10α-dihydroartemisinin-10-yl)ester (Ia-1), Its structural formula is as follows:
[0119]
[0120] Step 1) Synthesis of 4-((2-aminoethyl)amino)-3-nitrobenzenesulfonamide (hydrochloride) (1a)
[0121]
[0122] 3-nitro-4-chlorobenzenesulfonamide (2.36g, 0.01mol), 1-Boc-ethylenediamine (2.4g, 0.015mol), DIEA (3.87g, 0.03mol) were dissolved in 10mL of acetonitrile, 80 °C for 18 hours. After the reaction was completed, 10 mL of water was slowly added dropwise, and a yellow solid was precipitated. Filter with suction, and wash the precipitate three times with water / acetonitrile (V:V=1:1). Mix the filter cake with 30 mL of water, stir at 45°C for half an hour, filter with suction, wash the filter cake with water, and dry to obtain a ...
Embodiment 2
[0132] Example 2: Preparation of 4-((3-((4-(N-(2-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-( (4'-Chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl) Benzoyl)sulfonamido)-2-nitrophenyl)amino)propyl)amino)-4-oxobutanoic acid-(10α-dihydroartemisinin-10-yl)ester (Ia-2) , its structural formula is as follows;
[0133]
[0134] Synthetic steps are with embodiment 1;
[0135] M.p.141.7~141.9℃. LC-MS m / z:1217.1[M+Na] + . 1 H-NMR (600MHz, DMSO-d6) δ11.68(s, 1H), 11.37(s, 1H), 8.65(t, J=5.4Hz, 1H), 8.56(d, J=2.0Hz, 1H), 8.04(d,J=2.5Hz,1H),8.01(t,J=5.8Hz,1H),7.83(d,J=7.9Hz,1H),7.58–7.39(m,3H),7.34(d,J =8.4Hz, 2H), 7.05(t, J=10.3Hz, 3H), 6.68(dd, J=9.1, 1.9Hz, 1H), 6.39(dd, J=3.2, 1.8Hz, 1H), 6.18(d ,J=1.8Hz,1H),5.64(artemisinin characteristic peak d,J=9.7Hz,1H),5.50(s,1H),3.43–3.37(m,2H),3.19–3.10(m,2H) ,3.07(s,4H),2.75(s,2H),2.68–2.57(m,2H),2.41(t,J=6.7Hz,2H),2.30–2.24(m,1H),2.21–2.09(m ,6H),2.00–1.93(m,3H),1.78(dd,J=6.9,3.6Hz,1H),1.74–1.66(m,2...
Embodiment 3
[0136]Example 3: Preparation of 4-((4-((4-(N-(2-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-( (4'-Chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-yl)methyl)piperazin-1-yl) Benzoyl)sulfonamido)-2-nitrophenyl)amino)butyl)amino)-4-oxobutanoic acid-(10α-dihydroartemisinin-10-yl)ester (Ia-3) , its structural formula is as follows;
[0137]
[0138] Synthetic steps are with embodiment 1;
[0139] M.p.136.8~137.7℃. LC-MS m / z:1229.2[M+Na] + . 1 H-NMR(600MHz,DMSO)δ11.68(s,1H),11.40(s,1H),8.63–8.47(m,2H),8.04(d,J=2.5Hz,1H),7.90(t,J =5.6Hz,1H),7.80(d,J=9.2Hz,1H),7.54–7.47(m,3H),7.34(d,J=8.4Hz,2H),7.09–7.00(m,3H),6.68 (dd, J=9.1, 2.0Hz, 1H), 6.39(dd, J=3.2, 1.8Hz, 1H), 6.19(d, J=1.7Hz, 1H), 5.76(s, 1H), 5.64 (artemisia annua Prime characteristic peak d, J=9.7Hz, 1H), 5.52(s, 1H), 3.38(dd, J=13.1, 6.7Hz, 2H), 3.17–3.02(m, 6H), 2.75(s, 1H), 2.64–2.56(m,2H),2.38(t,J=7.1Hz,2H),2.31–2.11(m,8H),2.04–1.89(m,3H),1.85–1.74(m,1H),1.65– 1.45(m,8H),1.33–1.29(m,4H),1.27(artemisinin ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com