Spiro benzoanthracene-fluorene derivatives and their use in organic electronic devices, displays and lighting devices
A technology of organic electronic devices and electron transport layers, applied in lighting devices, organic semiconductor devices, electric solid devices, etc., can solve problems such as disturbing charge carrier mobility and reducing device performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0218] Reference will now be made in detail to the exemplary embodiments of the present invention, examples of which are illustrated in the accompanying drawings, wherein like reference numerals refer to like elements throughout. The exemplary embodiments are described below, by referring to the figures, in order to explain aspects of the present invention.
[0219] Here, when a first element is referred to as being formed or disposed "on" or "over" a second element, the first element may be directly disposed on the second element, or one or more other elements may be disposed on the first element. element and the second element. When a first element is referred to as being "directly formed or disposed on" or "directly formed or disposed on" a second element, there are no other elements interposed between the first element and the second element.
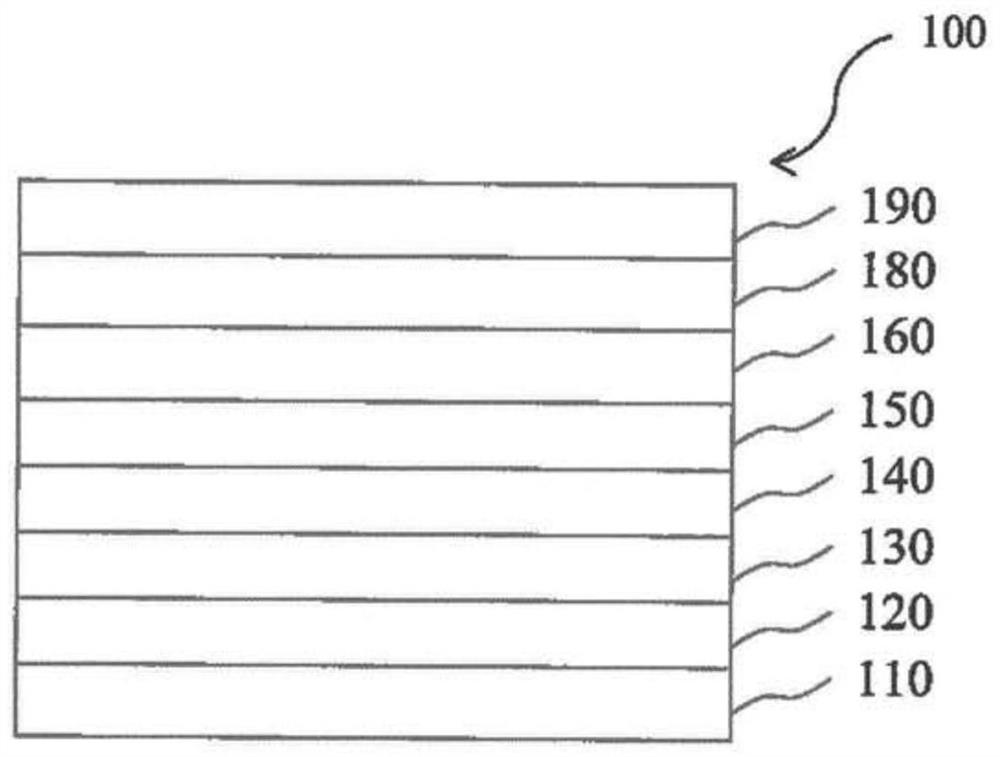
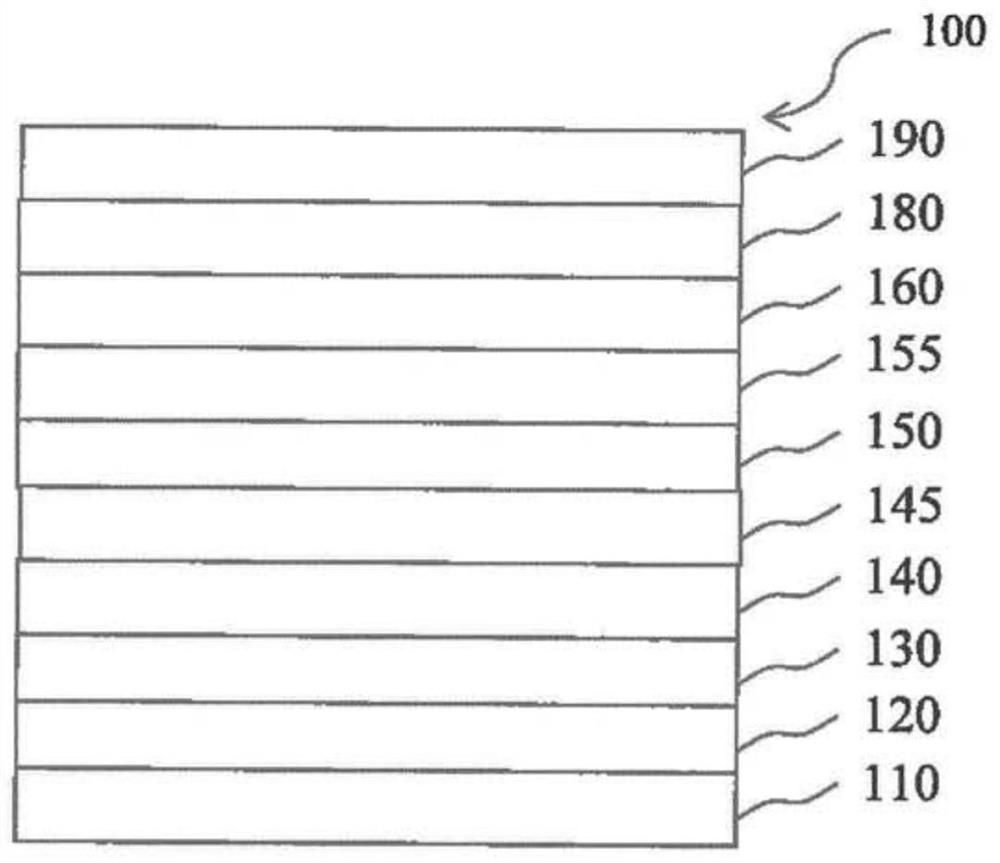
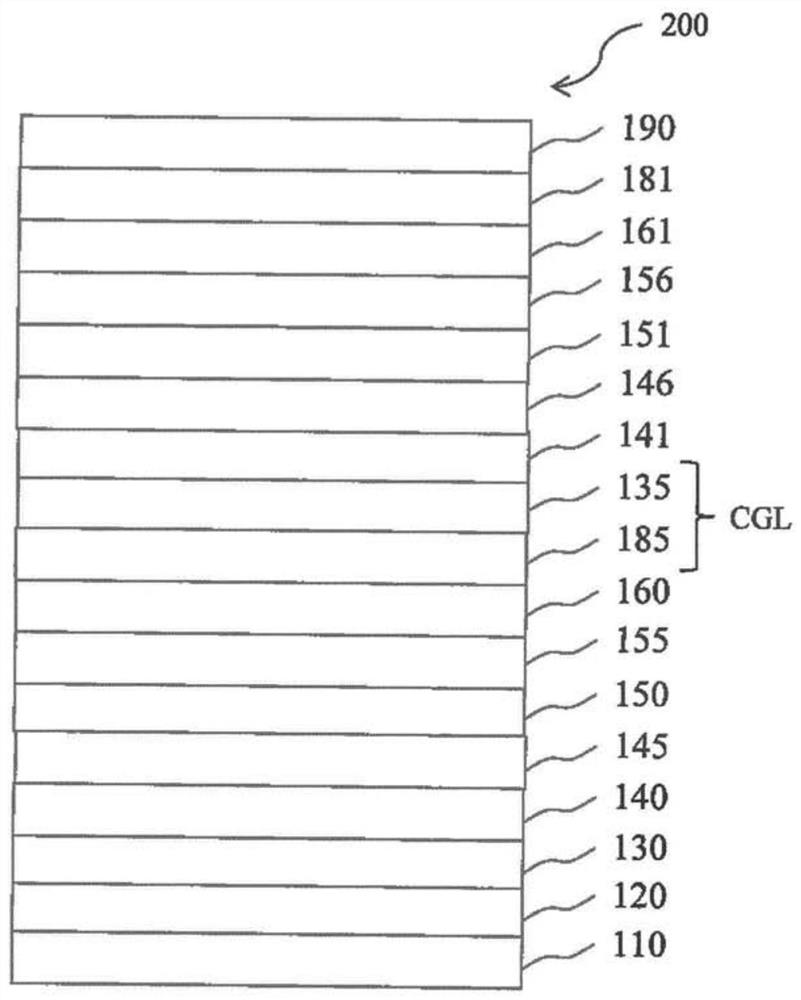
[0220] figure 1 is a schematic cross-sectional view of an organic light emitting diode (OLED) 100 according to an exemplary emb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com