Novel coronavirus, MERS and influenza A/B virus four-in-one rapid detection kit
A technology of influenza B virus and influenza A virus, applied in the biological field, can solve problems such as cross-reaction of detection kits and distinguishing new coronary pneumonia influenza
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of rabbit anti-new coronavirus S1 protein polyclonal antibody and rabbit anti-MERS virus S1 protein polyantibody
[0030] 1) immunity
[0031] (1) Take a healthy male New Zealand white rabbit with a body weight of about 2.5kg;
[0032] (2) Prepare the purified recombinant new coronavirus S1 protein with PBS (pH=7.0) solution to make 200μg / 850μl, add 150μl 501 immune adjuvant, and immediately after mixing, intramuscularly inject rabbit hind thighs, 500μl on the left and right sides, which is the initial immunization;
[0033] (3) On the 12th day after the initial immunization, use the same method to boost the immunization once;
[0034] (4) Booster immunization once every 10 days with the same method, a total of 3 booster immunizations;
[0035] (5) One week after each immunization, about 100 μl of blood was collected from the ear vein to measure the potency;
[0036] (6) One week after the last immunization, the serum titer should be determined ...
Embodiment 2
[0046] Example 2 Preparation of L-nCov, L-MERS, L-IFVA, L-IFVB and L-RIgG
[0047] Conjugation of ACE2 to carboxylated colored latex. Specific steps are as follows:
[0048] 1) The Rabbitanti-nCov S1 PAb prepared in Example 1 was coupled to the carboxy colored latex according to the EDC / NHS method described in the product manual of the carboxy colored latex to obtain L-nCov;
[0049] 2) Similarly, the Rabbit anti-MERS S1 PAb prepared in Example 1 was coupled to carboxyl colored latex to obtain L-MERS;
[0050] 3) Similarly, anti-IFVA monoclonal antibody (capture type) was coupled to carboxyl colored latex to obtain L-IFVA;
[0051] 4) Similarly, anti-IFVB monoclonal antibody (capture type) was coupled to carboxyl colored latex to obtain L-IFVB;
[0052] Similarly, rabbit IgG was also coupled to carboxyl green latex to obtain L-RIgG, which was used in the test strip quality control system.
Embodiment 3
[0053] Example 3 Preparation of a four-in-one rapid detection kit for novel coronavirus, MERS and influenza A / B
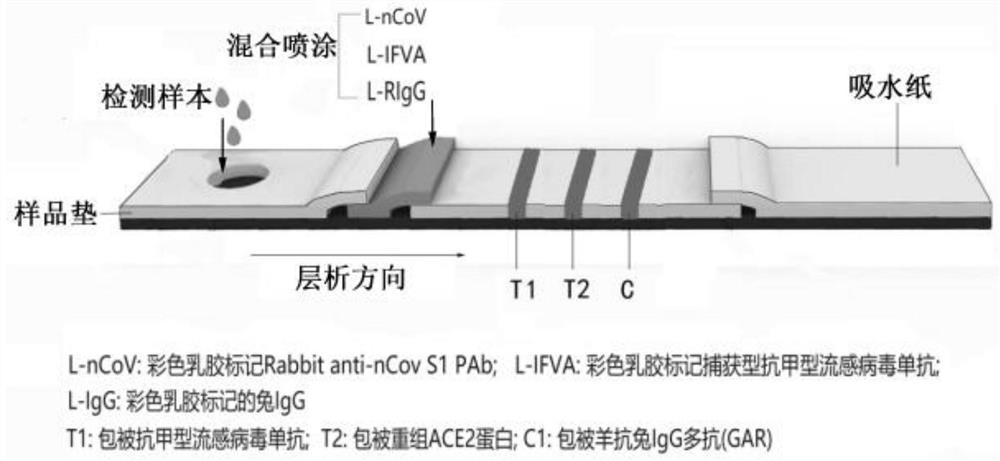
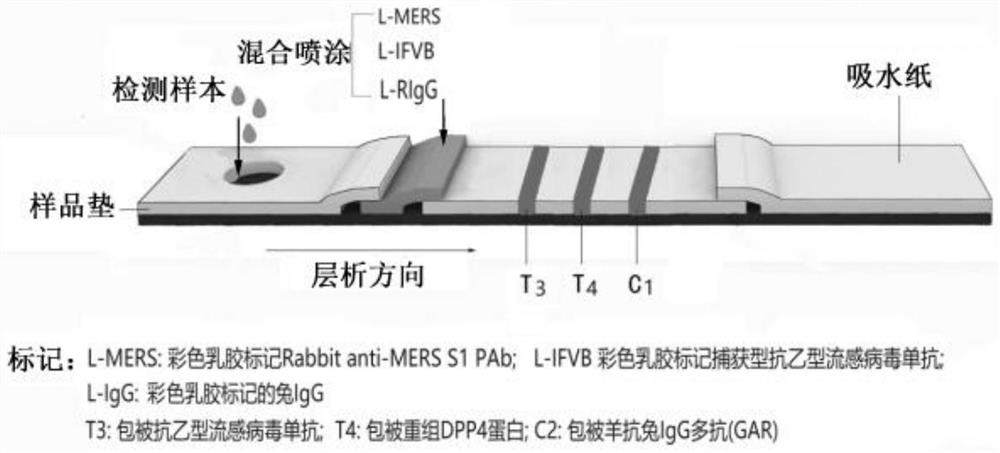
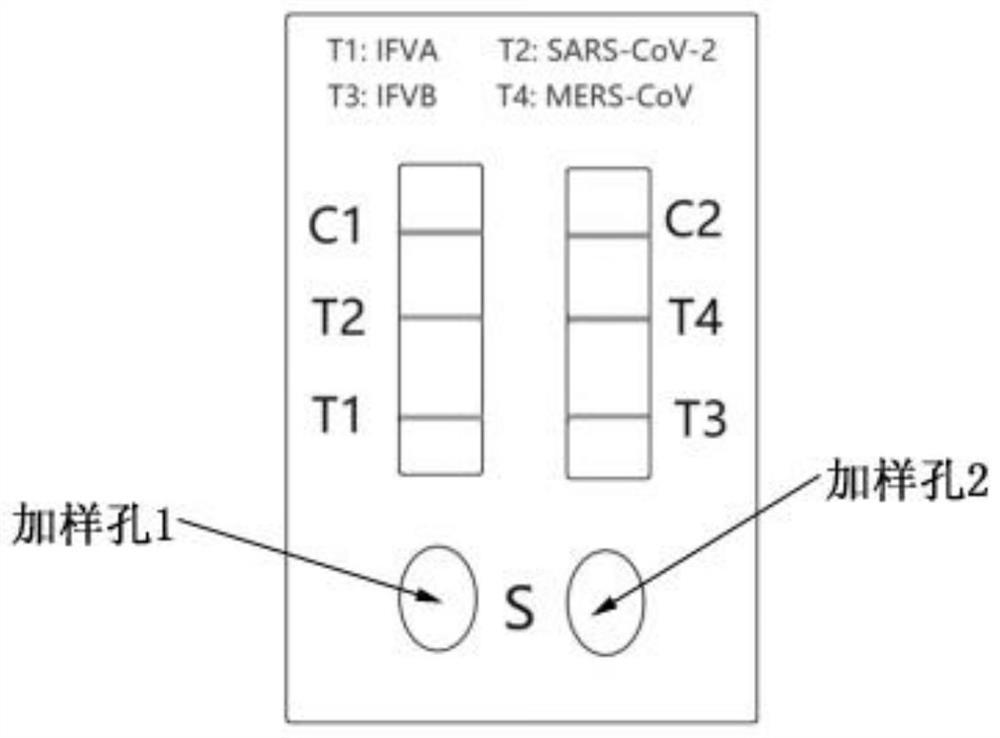
[0054] 1) The L-nCov, L-IFVA and L-RIgG prepared in the above Example 2 were mixed according to the mass ratio of 1:1:1, diluted with PBS (pH=7.0) solution to a total mass concentration of 0.6%, and sprayed on On the release pad of the test strip, dry at 37°C for 12 hours to form the release pad A, and use it for later use; similarly, mix L-MERS, L-IFVB and L-RIgG and spray on the release pad of the test strip, and dry at 37°C for 12 hours , is release pad B, spare.
[0055] 2) Dilute anti-IFVA monoclonal antibody (detection type), recombinant human ACE2 protein and goat anti-rabbit IgG polyclonal antibody (GAR) to 0.5mg / mL with coating diluent (150mM PB, pH 7.4), and apply to membrane Liquid volume of 30 μL / 30 cm was evenly sprayed and drawn on the T1, T2 detection line area and C1 control line area of the nitrocellulose membrane, dried at 37°C for 12 hours, se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com