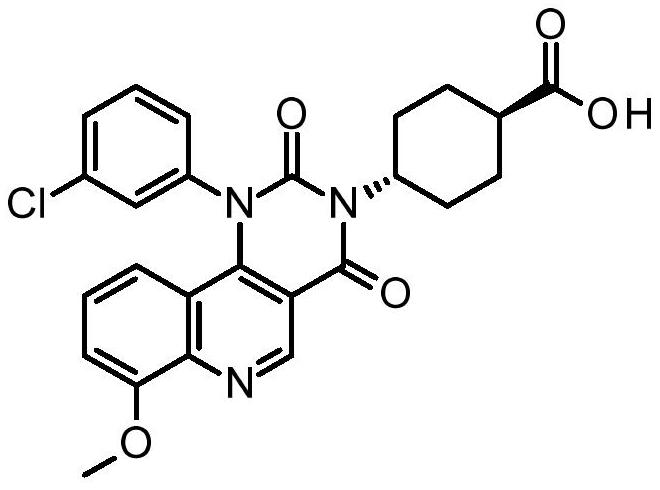
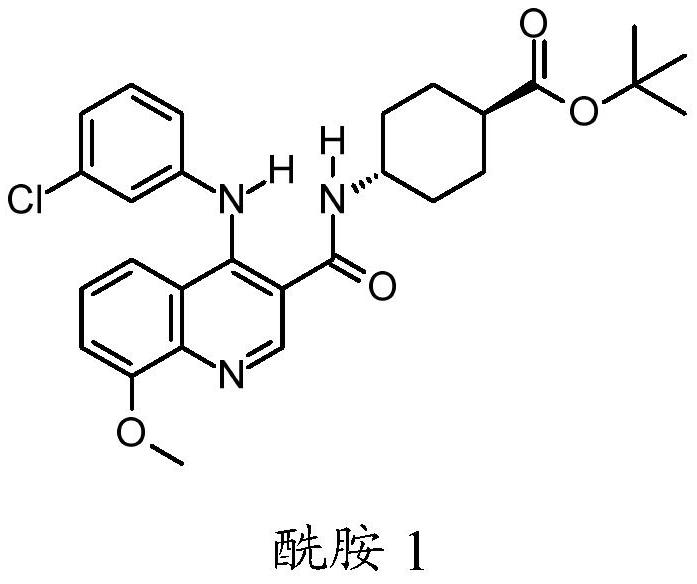
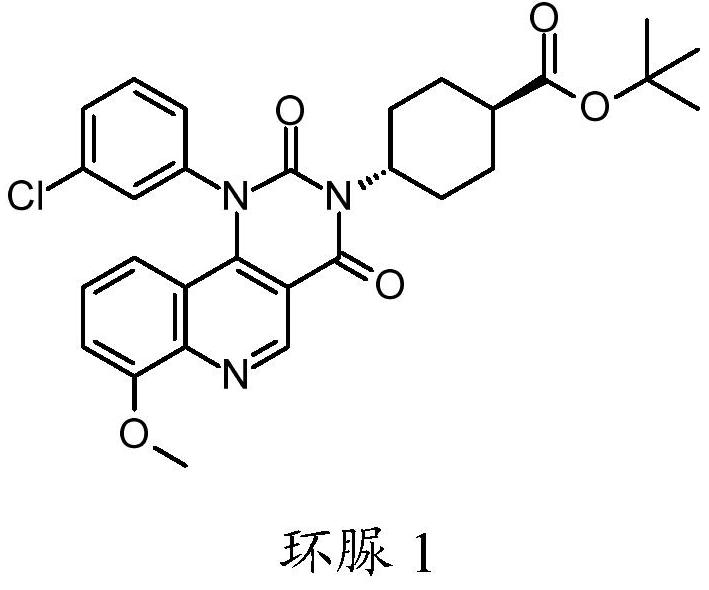
Quinoline derivatives, pharmaceutically acceptable salts thereof and methods of use thereof
A pharmacy and drug technology, applied to quinoline derivatives, can solve problems such as weak affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0083] Synthesis of compound I and its HCl salt
[0084] step 1:
[0085] To a 40 mL reaction vial was added ethyl 4-chloro-8-methoxyquinoline-3-carboxylate (1.25 g, 4.7 mmol), m-chloroaniline (0.69 g, 5.4 mmol, 1.15 equiv), 1-butanol (10 mL) and acetic acid (0.3 mL). The mixture was stirred at 95°C for 5 hours, IPC LCMS indicated the reaction was complete. 1-Butanol was removed in vacuo, and the mixture was diluted with ethyl acetate (75 mL). The organic layer was washed with saturated aqueous sodium bicarbonate (25 mL), and concentrated to dryness. The residue was purified by flash column chromatography eluting with DCM / ethyl acetate (1:0 to 1:1) to give the ester intermediate 4-(3-chloroanilino)-8-methoxy-quinone Ethyl pheno-3-carboxylate (1.05 g). Yield 62%. LCMS m / e 357.1(M+1) + ; 1 H NMR, 300MHz (CDCl 3 )δ1.45(t,3H),4.08(s,3H),4.44(q,2H),6.84(br d,1H),6.99(t,1H),7.04(dd,1H),7.05(d, 1H), 7.16(t,1H), 7.20s,1H), 7.22(dd,1H), 9.29(s,1H), 10.25(s,1H)ppm.
[0086] S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com