Spirooxazine photochromic compound changing from colorless to blue as well as preparation method and application of spirooxazine photochromic compound
A photochromic and compound technology, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve problems such as instability of the open ring state
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
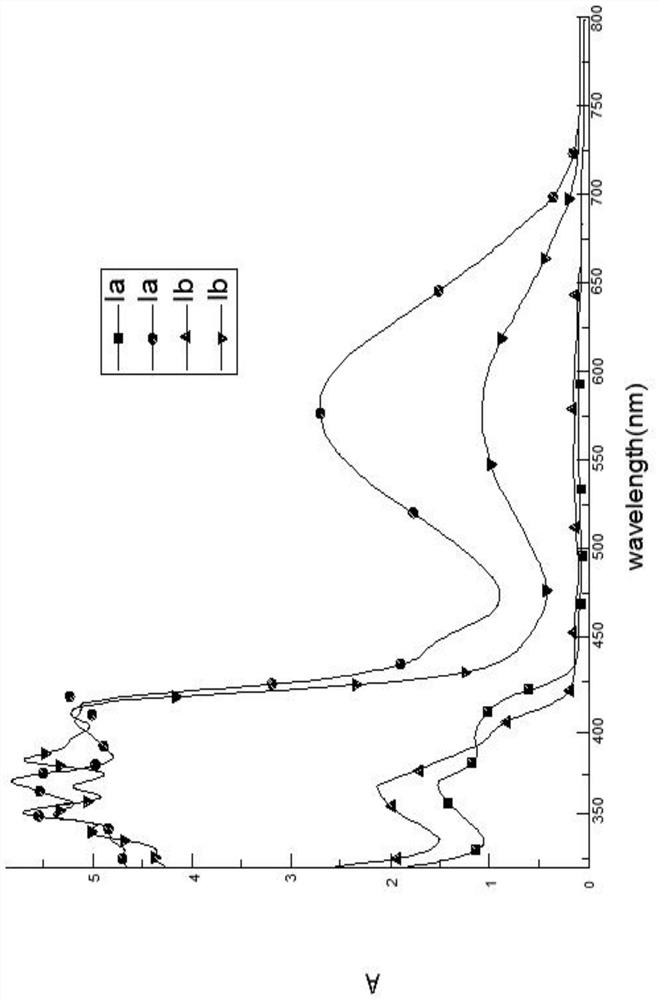
[0035] Example 1: Synthesis of a colorless to blue spirooxazine photochromic compound Ia:
[0036] The first step: the synthesis of M2a, the reaction formula is as follows:
[0037]
[0038] In a 250ml four-neck flask, add fluorenone (9.003g, 0.050mol), then add 100ml of glacial acetic acid solvent, magnetically stir, heat to 30℃, dissolve completely, then slowly add NBS (10.680g, 0.060 mol). After the addition, the reaction was continued at 30°C for 3h (TCL monitoring). After the reaction, the reaction solution was poured into a large amount of water. The product was separated out, filtered with suction, washed with water, and recrystallized with petroleum ether / ethyl acetate. Yield: 76%.
[0039] Step 2: Synthesis of M3a, the reaction formula is as follows:
[0040]
[0041] In a 100ml four-necked flask, add 80ml of deionized water, under magnetic stirring, add NaOH (1.200g, 0.030mol), heat to 100℃ to dissolve completely, then slowly add M2a (5.159g, 0.020mol), the addition is comp...
Embodiment 2
[0052] Example 2 Synthesis of a colorless to blue spirooxazine photochromic compound Ib:
[0053] The first step: the synthesis of M2b, the reaction formula is as follows:
[0054]
[0055] In a 100ml four-neck flask, add fluorenone (9.003g, 0.050mol), then add 100ml of glacial acetic acid solvent, magnetically stir, constant temperature at 25℃, dissolve completely, then slowly add NBS (8.890g, 0.050 mol). After the addition is complete, continue to stir the reaction at 25°C for 4h (TCL monitoring). After the reaction is complete, pour the reaction solution into a large amount of water. The product is separated out, filtered with suction, washed with water, and recrystallized with petroleum ether / ethyl acetate. Yield: 55%.
[0056] Step 2: Synthesis of M3b, the reaction formula is as follows:
[0057]
[0058] In a 100ml four-neck flask, add 80ml deionized water and 12ml ethanol. Under magnetic stirring, add NaOH (1.160g, 0.029mol), heat to 90℃ to dissolve completely, then slowly add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com