Baculovirus expression system and construction method and application thereof
A baculovirus and expression system technology, applied in the field of protein expression, can solve problems such as time-consuming, labor-intensive, reduced work efficiency, and cumbersome operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
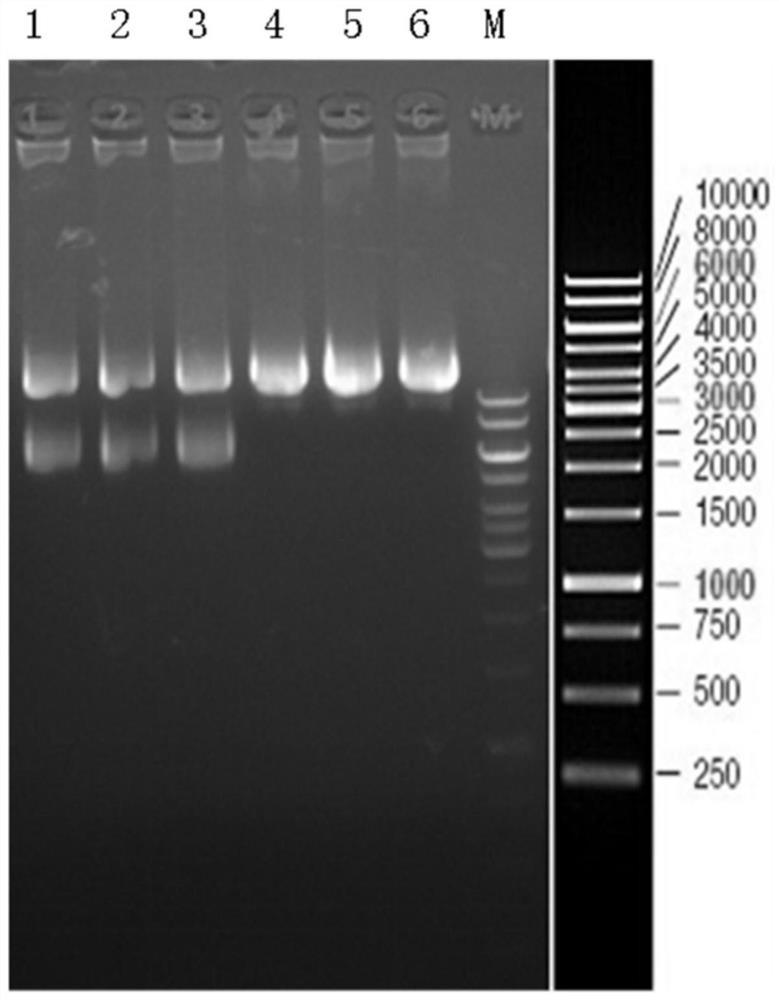
Examples
Embodiment 1
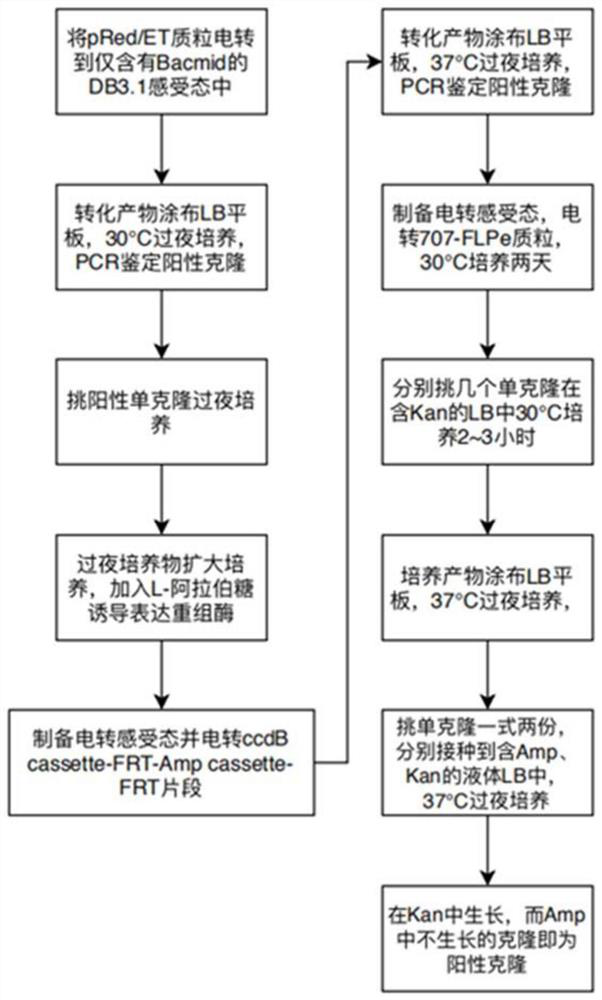
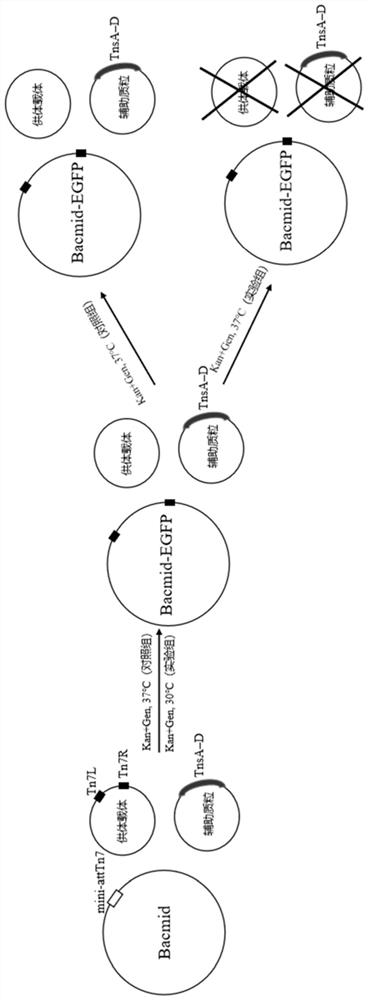
[0061] 1. Construction of baculovirus expression vector system
[0062] The common replicons of pFastBac and pHelper were replaced by the temperature-sensitive pSC101ori replicons by the Gibson cloning method, and then the ccdA expression cassette was inserted on the basis of the temperature-sensitive helper plasmid to construct optimized donor vectors pFastBac(ts) and Helper vector pHelper(ts+ccdA).
[0063] Such as figure 1 As shown, the homologous recombinase expression plasmid pRed / ET (tetracycline-resistant Tc) was electrotransferred into the competent DB3.1 containing only the Bacmid vector (Kana-resistant Kan), added to SOC culture and incubated at 30°C for 1 hour .
[0064] The resuscitated culture products were spread on LB plates containing Tc+Kan antibiotics, cultured at 30°C for two days, and positive clones containing both pRed / ET and Bacmid vectors were identified by PCR.
[0065] Pick positive clones and inoculate them into LB liquid medium containing Tc+Kan ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com