Polypeptide and use thereof in anti-glomerular basement membrane disease
An amino acid and hydrophobic amino acid technology, applied in the field of immunotherapy, can solve serious problems, lack of specific treatment plan, high cost and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. Design and synthesis of polypeptides
[0039] Identification of core epitopes
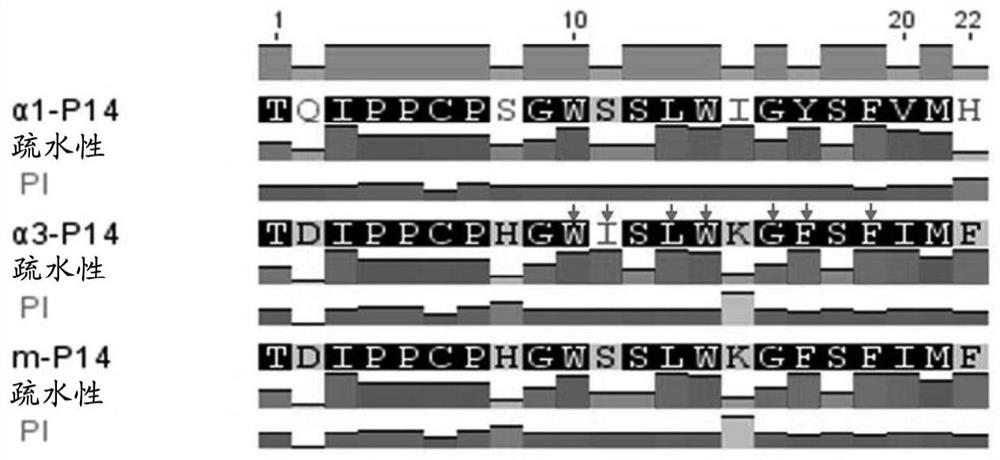
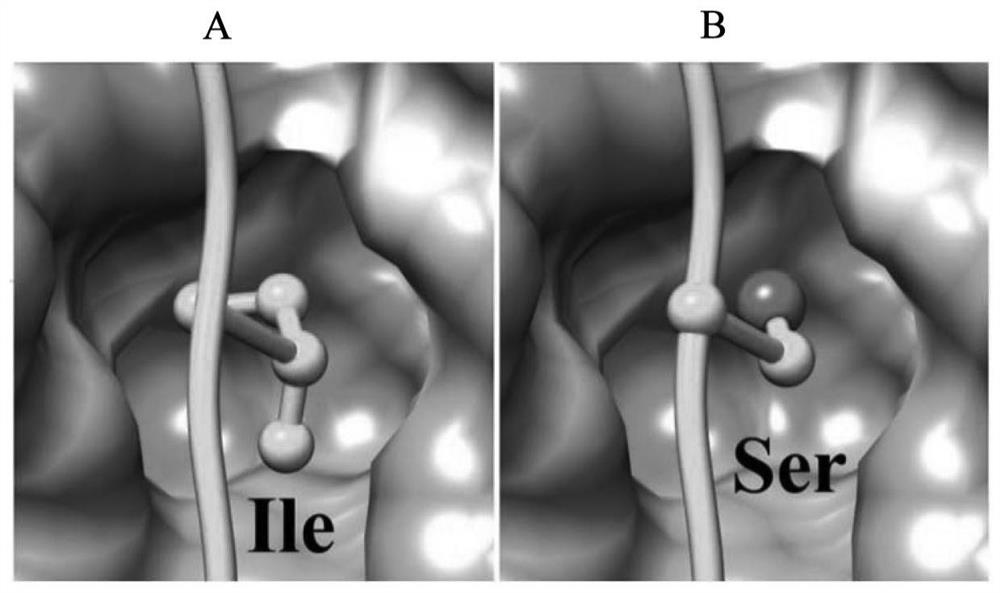
[0040] Studies have shown that the α3-P14 peptide (α3 127-148 : TDIPPCPHGWISLWKGFSFIMF; SEQ ID NO: 1) includes an antigenic epitope recognized by T cells and is closely related to the development of anti-GBM disease (Hu SY, et al., supra). Immunization of animals with α3-P14 peptide can induce anti-GBM disease, and the identification of key amino acids related to pathogenicity in α3-P14 peptide shows that when tryptophan at position 136 (W136) in α3-P14 peptide , isoleucine at position 137 (I137), leucine at position 139 (L139), tryptophan at position 140 (W140) or phenylalanine at position 143 (F143) are mutated, Immune animals no longer get sick. It can be seen that the motif WIxLWxxF composed of the above amino acids is the key amino acid motif for the α3-P14 peptide to induce anti-GBM disease.
[0041] In addition, a key site in the α3-P14 peptide that binds to the HLA-D...
Embodiment 2
[0049] Example 2. Pathogenicity Research of Polypeptides
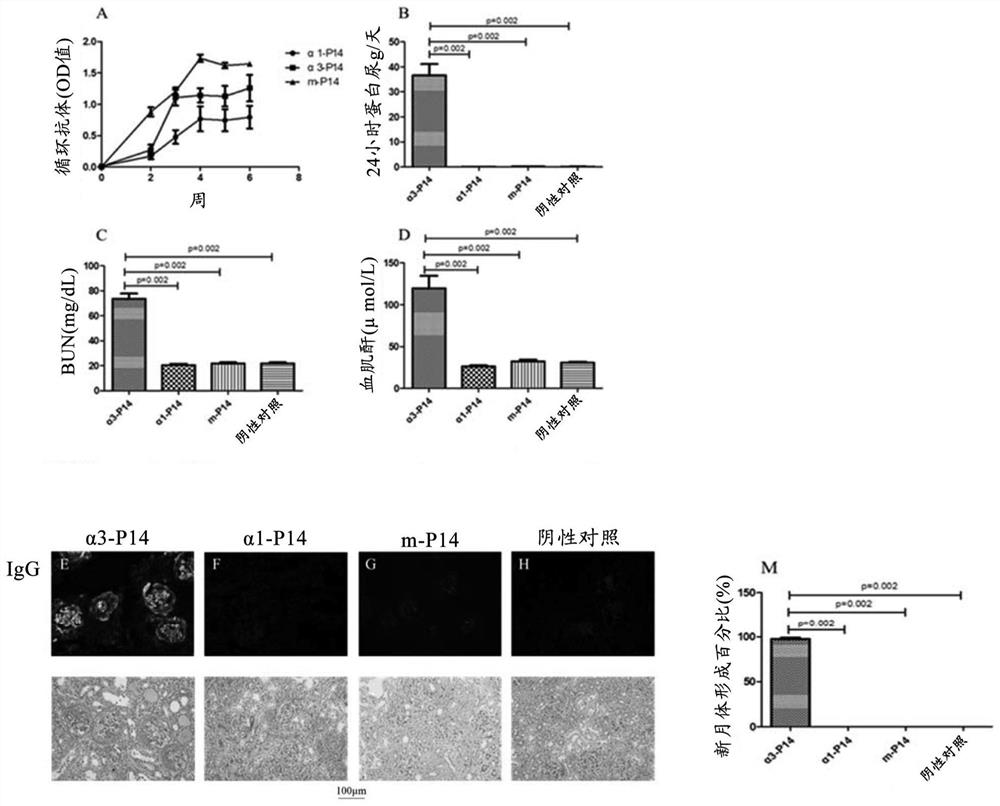
[0050] The pathogenicity of modified m-P14 polypeptides against GBM disease was first investigated. Refer to the previous establishment method of anti-GBM rat animal model (Hu SY et al.J.Cell.Mol.Med.Vol 21, No 9,2017pp.2117-2128), detect α3-P14 polypeptide and m-P14 polypeptide Whether anti-GBM disease can be induced after immunization of animals. Briefly, WistarKyoto (WKY) rats (female, 4 week age, each group n=6). Immunization was performed with a single rear footpad injection at a dose of 200 μg / kg of the polypeptide. The negative control group was injected with PBS only. Hematuria samples were collected before immunization and weekly after immunization. Animals were sacrificed at the end of the 6th week after immunization, and kidney tissues were collected for pathological observation.
[0051] The results showed that, two weeks after immunization, antibodies against corresponding immunogens were produced in...
Embodiment 3
[0053] Example 3.m-P14 polypeptide prevents and treats the effect of anti-GBM disease
[0054] This example studies the effect of m-P14 polypeptide in the prevention and treatment of anti-GBM disease.
[0055] experimental design
[0056] All WKY rats were injected with α3-P14 polypeptide into the rear foot pad once (day 0) to induce anti-GBM disease, and the dose was 200 μg / kg subcutaneously. After immunization, the rats were divided into prevention group (treatment started from day 0), treatment group (treatment started after the hematuria / proteinuria symptoms of anti-GBM disease appeared in experimental rats), disease group (no intervention after immunization), and At the same time, a negative control group (only injected with PBS) was set up, with 6 animals in each group. For specific experimental procedures, see Figure 4 .
[0057] Prevention group: divided into high-dose group (30 mg / kg / time) and low-dose group (10 mg / kg / time) according to the dosage of m-P14 poly...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap