Method for preparing 2-(methylsulfinyl)benzoic acid
A technology of methyl sulfinyl and benzoic acid, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of cumbersome reaction steps, high temperature, unstable corrosiveness, etc., and avoid toxic reagents. The effect of use, yield promotion, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
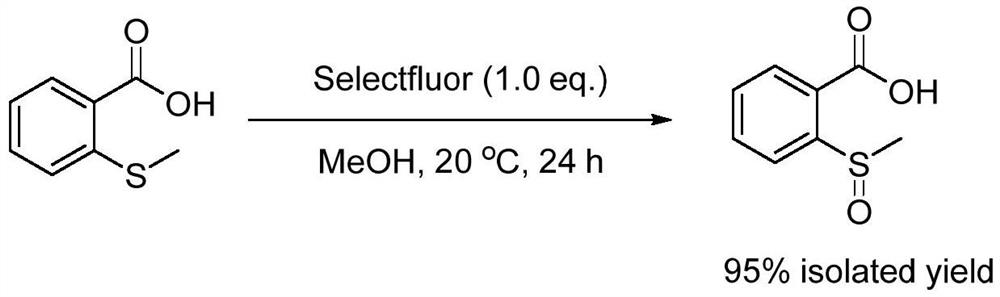
[0018] Specific Example 1: Add methanol (30mL), 2-(methylthio)benzoic acid (6mmol, 1.01g), 1-chloromethyl-4-fluoro-1,4- Diazabicyclo[2.2.2]octane bis(tetrafluoroborate) salt [Selectfluor] (6mmol, 2.13g), the reaction temperature was controlled at 20 degrees Celsius, and the reaction was vigorously stirred for 24 hours. After the reaction was completed, the reaction solution was filtered, concentrated, and dried to obtain the target product 2-(methylsulfinyl)benzoic acid (1.05 g, 95%). The equations involved in the reaction are as follows:
[0019]
[0020] The NMR data and mass spectrum data of the target product 2-(methylsulfinyl)benzoic acid are as follows:
[0021] 1 H NMR (300MHz, DMSO) δ8.15–8.13(m,1H),8.07–8.04(m,1H),7.92(t,J=7.4Hz,1H),7.67(t,J=7.5Hz,1H) ,2.75(s,3H).
[0022] 13 C NMR (75MHz, DMSO) δ167.01, 150.76, 134.23, 131.33, 130.87, 127.85, 123.95, 44.41.
[0023] LCMS(ESI,m / z):185.00[M+H] + .
specific Embodiment 2
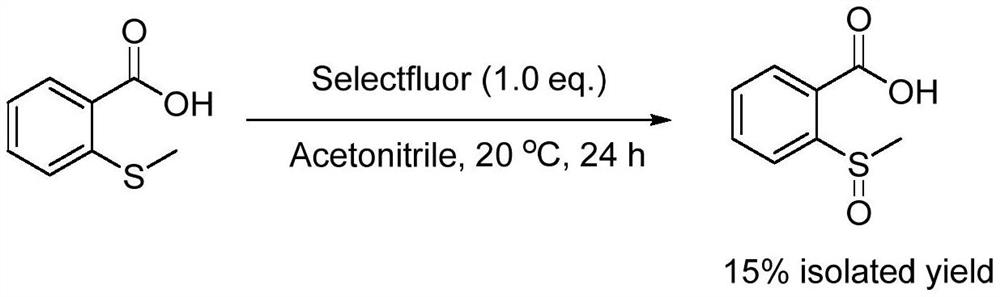
[0024] Specific Example 2: Add acetonitrile (30mL), 2-(methylthio)benzoic acid (6mmol, 1.01g), 1-chloromethyl-4-fluoro-1,4- Diazabicyclo[2.2.2]octane bis(tetrafluoroborate) salt [Selectfluor] (6mmol, 2.13g), the reaction temperature was controlled at 20 degrees Celsius, and the reaction was vigorously stirred for 24 hours. After the reaction, the reaction solution was filtered, concentrated, and dried to obtain the target product 2-(methylsulfinyl)benzoic acid (0.17 g, 15%). The equations involved in the reaction are as follows:
[0025]
specific Embodiment 3
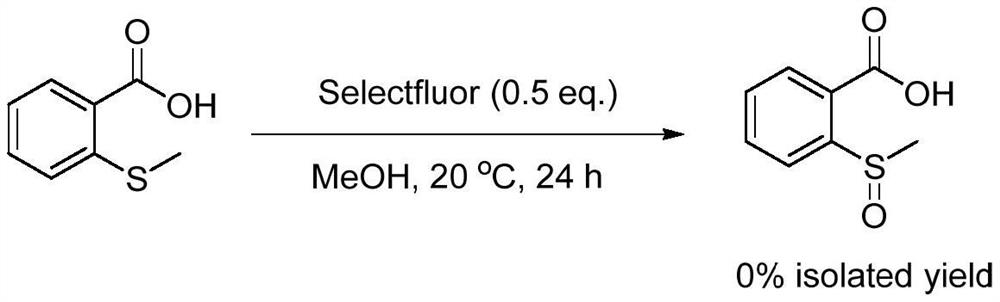
[0026] Specific Example 3: Add methanol (30mL), 2-(methylthio)benzoic acid (6mmol, 1.01g), 1-chloromethyl-4-fluoro-1,4- Diazabicyclo[2.2.2]octane bis(tetrafluoroborate) salt [Selectfluor] (3mmol, 1.06g), the reaction temperature was controlled at 20 degrees Celsius, and the reaction was vigorously stirred for 24 hours. After the reaction was completed, the reaction solution was filtered, concentrated, and dried in sequence, but the target product 2-(methylsulfinyl)benzoic acid was not isolated. The equations involved in the reaction are as follows:
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com