Method for increasing content of intracellular heme in escherichia coli
A technology of Escherichia coli and recombinant Escherichia coli, which is applied in the field of metabolic engineering and can solve problems such as increased cost and inconvenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
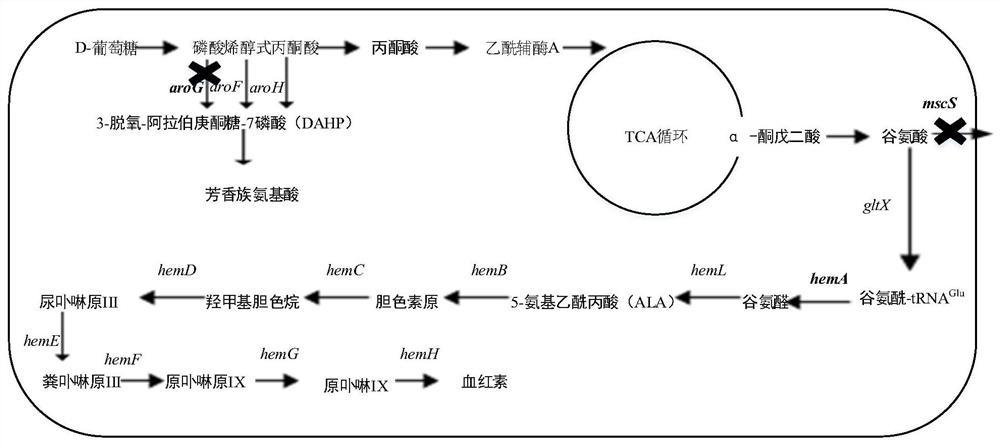
[0035] According to SEQ ID NO.1, each 500bp homology arm of its upstream and downstream was designed, through the method of Red homologous recombination (see Datsenko KA.One-step inactivation of chromosome genes in Escherichiacoli K-12 using PCR products.Proceedings of the specific steps National Academy of Sciences of the United States of America, 2000,97(12):6640-6645)) knock out the mscS gene in Escherichia coli BL21, after sequencing verification, obtain the genetically engineered bacterium that knocks out the mscS gene, and name it for WT-M.
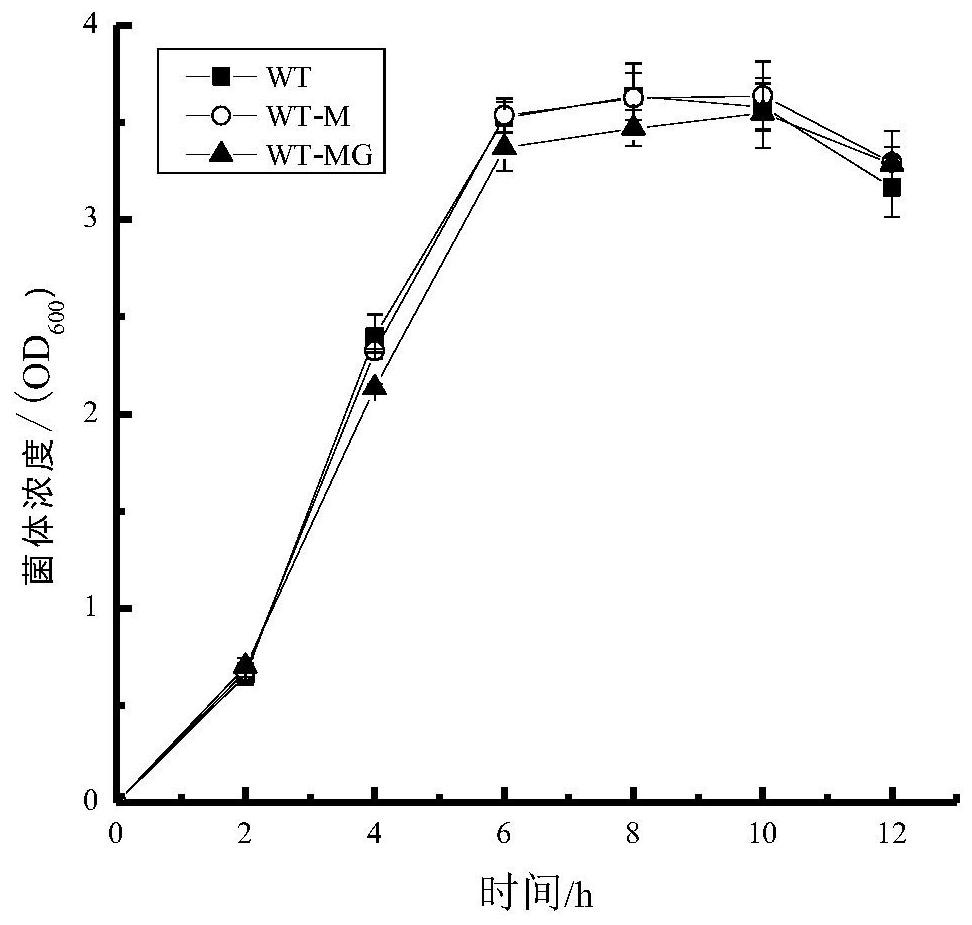
[0036] Inoculate the genetically engineered bacteria WT-M into LB medium, activate overnight at 37°C, 200r / min, transfer 500μL of a certain concentration of bacterial solution to 50mL of LB medium the next day, and incubate at 37°C, 200r / min Under culture, measure the bacterial concentration in real time, and make a growth curve, such as figure 2 shown.
[0037] Inoculate strain WT into LB medium, activate overnight at 37°C, 200r...
Embodiment 2
[0041] According to SEQ ID NO.2, the upstream and downstream 50bp homology arms were designed, and the aroG gene was knocked out on the basis of WT-M through the Red homologous recombination method. After sequencing verification, the genetic engineering of knocking out the aroG gene was obtained. bacterium, and named it as WT-MG, and obtained the genetically engineered bacterium.
[0042] Inoculate the genetically engineered bacteria WT-MG into LB medium, activate overnight at 37°C, 200r / min, and take 500μL of the activated bacterial solution the next day and transfer it to 50mL of LB medium, at 37°C, 200r / min Under culture, measure the bacterial concentration in real time, and make a growth curve, such as figure 2 As shown, there was no significant difference in the growth of genetically engineered bacteria WT-MG and WT.
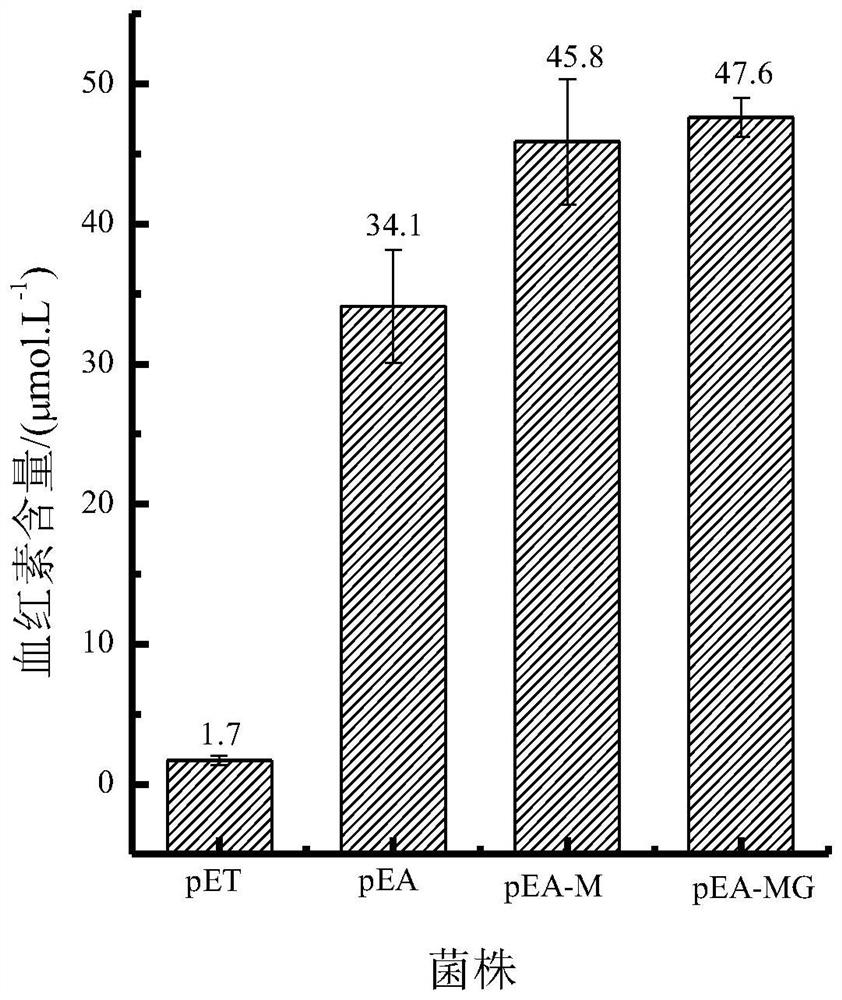
[0043] After culturing for 7 hours, the hemoglobin content was detected, and the hemoglobin content in WT-MG was 2.86 μmol / L.
Embodiment 3
[0045] The glutamyl-tRNA reductase hemA gene (nucleotide sequence shown in SEQ ID NO.3) was connected to the pET28a vector through HindⅢ and EcoR I to obtain the recombinant plasmid pET28a-hemA, and then the recombinant plasmid was transferred into JM109 , spread the bacterial solution on an LB plate, culture at 37°C until a single clone grows, pick a single clone and perform sequencing verification, and the correct verification is a positive transformant. Inoculate the positive transformants into LB liquid medium, cultivate for 8-12 hours, extract the recombinant plasmid pET28a-hemA from the bacterial liquid, introduce the recombinant plasmid into the bacterial strain WT-M obtained in Example 1, and obtain the engineering bacterium pEA-M, The hemoglobin concentration was detected by fluorescence method, and the results showed that the hemoglobin content of pEA-M reached 45.8 μmol L -1 .
[0046] The pET28a plasmid was transformed into BL21 with no load, and the engineered ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com