New applications of sorafenib, regorafenib and their analogs or derivatives
A drug resistance technology, applied in the field of medicine, can solve the problems of high cost of bone marrow transplantation and lack of good therapeutic drugs, and achieve the effect of good tolerance of clinical patients, light side effects and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the establishment of two common drug-resistant cell models (HEL PE , HEL RE ).
[0046] 1. Materials and methods
[0047] 1. Cell line
[0048] HEL (Human erythroleukemia cell line) and drug-resistant HEL cells were cultured in RPMI medium (Gibco) containing 20% heat-inactivated fetal bovine serum (Gibco) and 1% penicillin / streptomycin (Gibco).
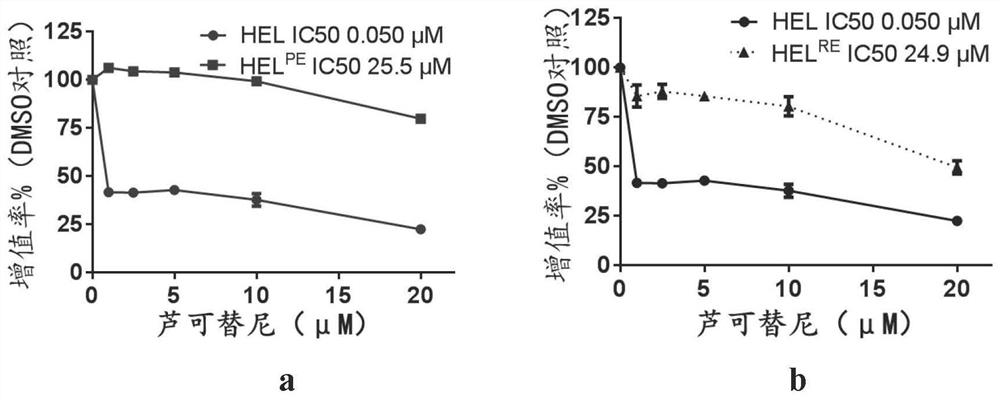
[0049] HEL PE The model is the HEL-persistent model, which is constructed by treating HEL primitive cells with a high concentration of ruxolitinib that exceeds the IC50 concentration of the primitive cells by 100 times, and the concentration we use is 2.0 μM. The solution was changed every two days, and the cells that could not tolerate it died quickly, and the amplified cells were DTP (drug-tolerant-persisters), which were difficult to be killed by anti-tumor drugs. Stable drug-resistant cells were obtained after 4-6 weeks. Another resistance model is HEL RE That is, the HEL-resistant model, the construc...
Embodiment 2
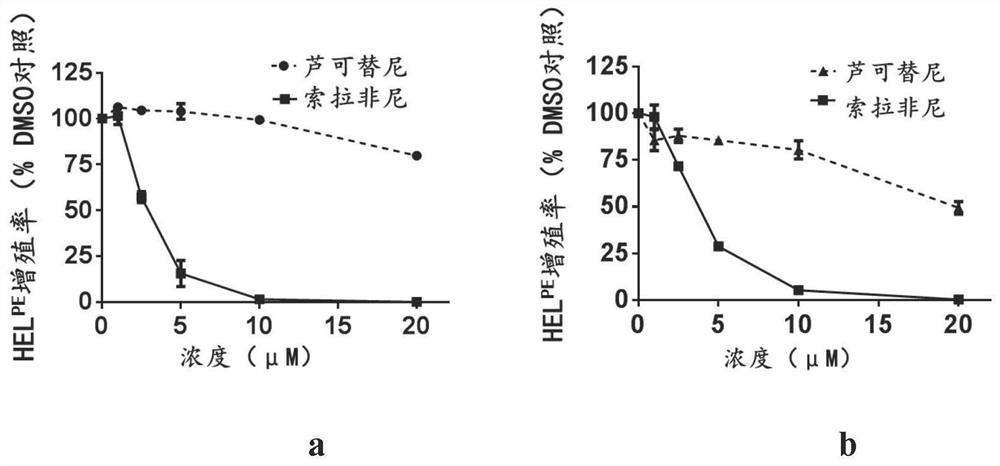
[0058] Example 2, Sorafenib can inhibit the proliferation of two drug-resistant cell lines
[0059] The method is to use increasing concentrations of Sorafenib to treat drug-resistant cell lines, through CellTiter-Lumi TM Luminescence method is used to detect the proliferation of cells, the method is the same as in Example 1, and the results are shown in figure 2 .
[0060] The results show, figure 2 a reflects that in the ruxolitinib-resistant cell model HEL-persistent, the IC50 value of sorafenib is 2.80 μM, which is 1 / 9 of the ruxolitinib IC50 value of 25.5 μM, figure 2b reflects that in the ruxolitinib-resistant cell model HEL-resistant, the IC50 value of sorafenib is 3.56 μM, which is 1 / 7 of the ruxolitinib IC50 value of 24.9 μM. This shows that sorafenib can inhibit the proliferation of ruxolitinib-resistant cells, and this effect is enhanced with the increase of concentration.
Embodiment 3
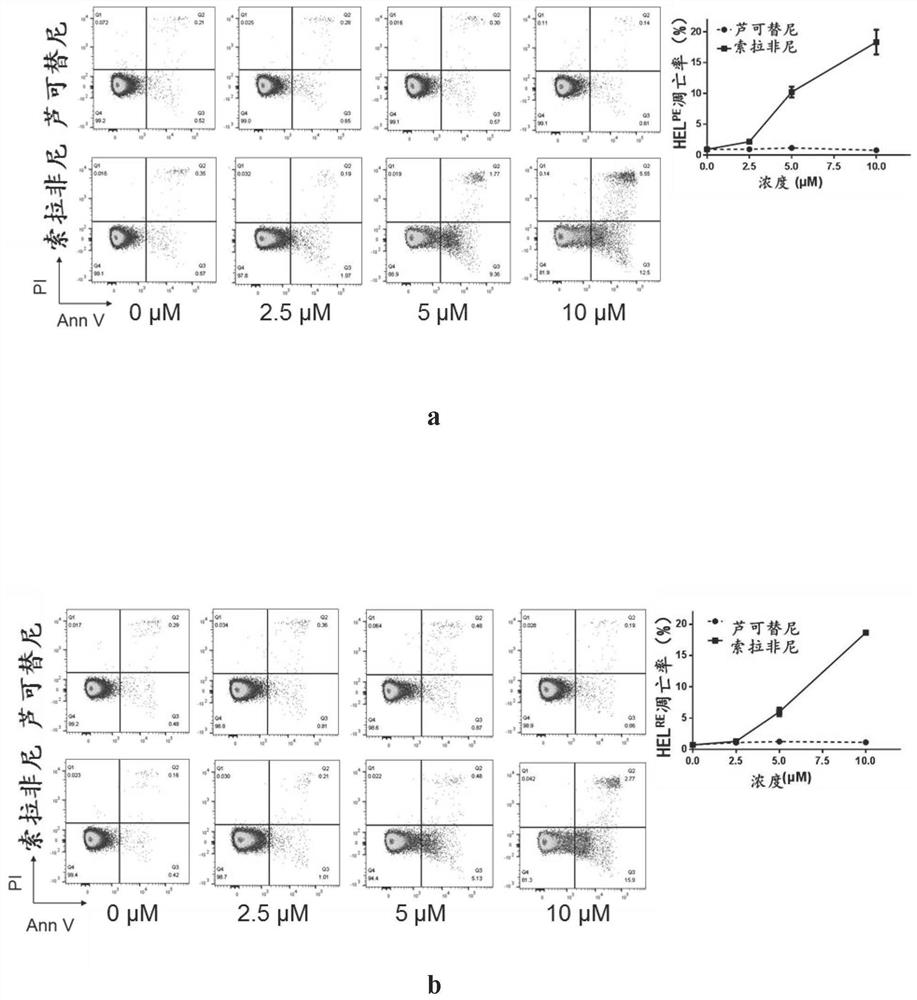
[0061] Example 3, Sorafenib can promote the apoptosis of two drug-resistant cell lines
[0062] 1. Materials and methods
[0063] 1. Cell lines and inhibitors are the same as in Example 1.
[0064] 2. Apoptosis detection
[0065] In order to detect the pro-apoptotic effect of the inhibitor, the drug-resistant cell line was treated with increasing concentrations of Sorafenib for 24 hours (concentration: 0, 2.5, 5, 10 μM), and DMSO was supplemented to the same amount. Three parallel repeat groups were set up, and the apoptosis of cells was detected by flow cytometry after AnnexinV-PI staining.
[0066] Apoptotic rate calculation formula: Apoptotic rate = ratio of early apoptotic cells (AnnexinV+ / PI-) + ratio of late apoptotic cells and necrotic cells (AnnexinV+ / PI+).
[0067] 2. Results analysis
[0068] image 3 In a, the drug concentration (average apoptosis rate) of ruxolitinib treatment group is: 0 μM (0.91%), 2.5 μM (0.920%), 5 μM (1.11%), 10 μM (0.740%); Sorafenib tre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com