Regorafenib related substances as well as preparation method and application thereof
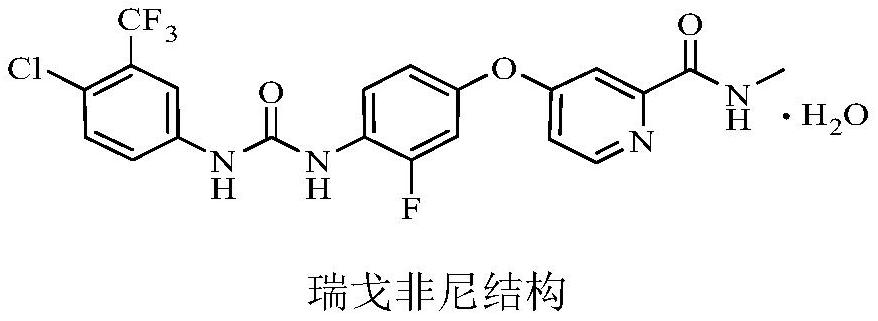
A related substance, regorafenib technology, applied in the field of regorafenib related substances and its preparation, can solve the problem of no literature report on substance D, and achieve high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
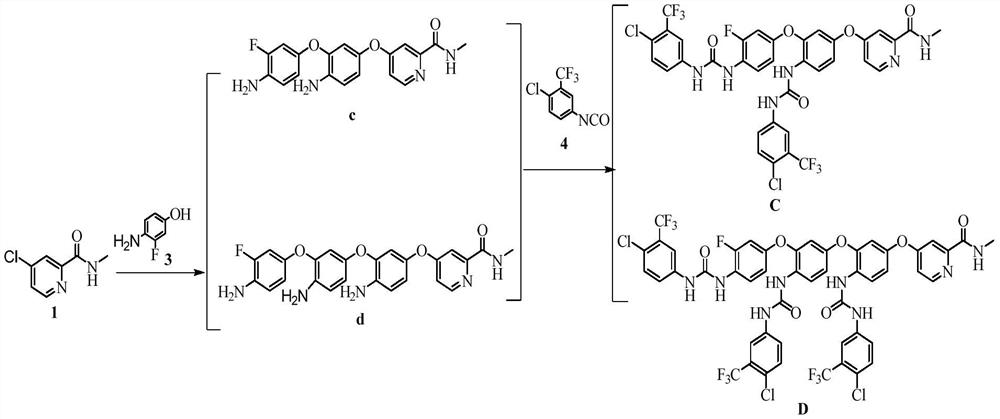
[0027] Embodiment 1: the preparation of compound c and compound d
[0028] Experiments were carried out on the effect of reaction temperature during the preparation of compound c and compound d. The experimental design is as follows:
[0029] Weigh 5.00g (0.029mol) 4-chloro-N-methylpyridine-2-carboxamide (compound 1) and 7.45g (0.059mol) 4-amino-3-fluorophenol (compound 3) and 6.58g (0.059 mol) Potassium tert-butoxide, add to 50mL N,N-dimethylformamide, heat, react for 4h, after the reaction is completed, add purified water, extract with ethyl acetate, combine the organic phases, concentrate under reduced pressure, and separate and purify by column chromatography (Silica gel: 200-300 mesh; eluent: V (ethyl acetate): V (n-hexane) = 1:4, 1:3, 1:2, 1:1) Concentrate to dryness to obtain compound c and compound d .
[0030] Table 1 The influence of different experimental temperatures on the reaction
[0031]
[0032] From the above experimental data, it can be seen that when...
Embodiment 2
[0036] Embodiment 2: the preparation of compound c and compound d
[0037] Experiments were performed on the effect of the amount of starting materials during the preparation of compound c and compound d. The experimental design is as follows:
[0038] Weigh 4-chloro-N-methylpyridine-2-carboxamide (compound 1), 4-amino-3-fluorophenol (compound 3) and potassium tert-butoxide, add to 50mL N,N-dimethylformamide Amide, heated to 120±5°C, reacted for 4h, after the reaction was completed, added purified water, extracted with ethyl acetate, combined the organic phases, concentrated under reduced pressure, separated and purified by column chromatography (silica gel: 200-300 mesh; eluent: V (Ethyl acetate): V (n-hexane) = 1:4, 1:3, 1:2, 1:1) concentrated to dryness to obtain compound c and compound d.
[0039] The influence of the amount of table 2 compound 1, compound 3 and potassium tert-butoxide on reaction
[0040]
[0041]
[0042] It can be seen from the above data that ...
Embodiment 3
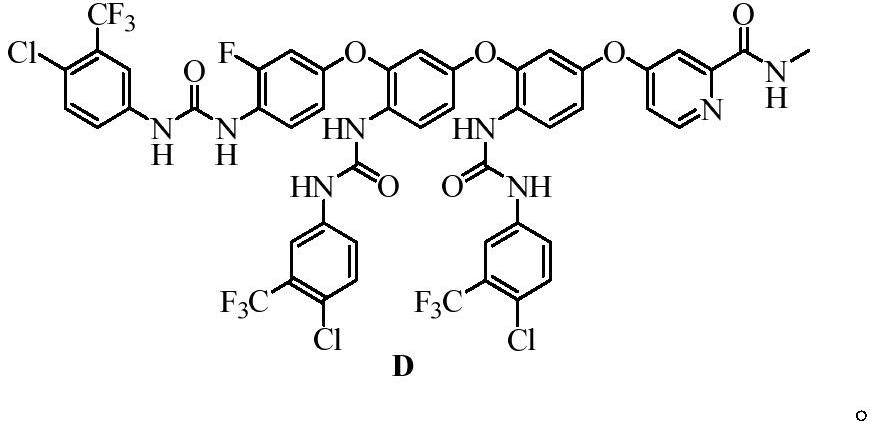
[0043] Embodiment 3: the preparation of compound C and compound D
[0044] Experiments were performed on the effect of the amount of starting materials during the preparation of Compound C and Compound D. The experimental design is as follows:
[0045]Weigh compound c and compound d obtained in Example 2-2 and add them to 5 mL of dichloromethane, add dropwise a dichloromethane solution containing 4-chloro-3-(trifluoromethyl)phenylisocyanate (compound 4), and react at room temperature 15h, concentrated under reduced pressure, separated and purified by column chromatography (silica gel: 200-300 mesh; eluent: V (n-hexane): V (ethyl acetate) = 1:5, 1:4, 1:3, 1: 2, 1:1), concentrated to dryness to obtain the product compound C and compound D.
[0046] Table 3 The impact of the amount of compound c, compound d and compound 4 on the reaction
[0047]
[0048] Compared with Example 3-2, the yield and purity of Example 3-3 are not much different, but there are a large number of i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com