In-vitro model of central nervous system poisoning and method for evaluating curative effect of heavy activator
A technology of central nervous system and activator, applied in the field of medicine, can solve the problems of inaccurate evaluation results, and achieve the effect of high evaluation accuracy and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
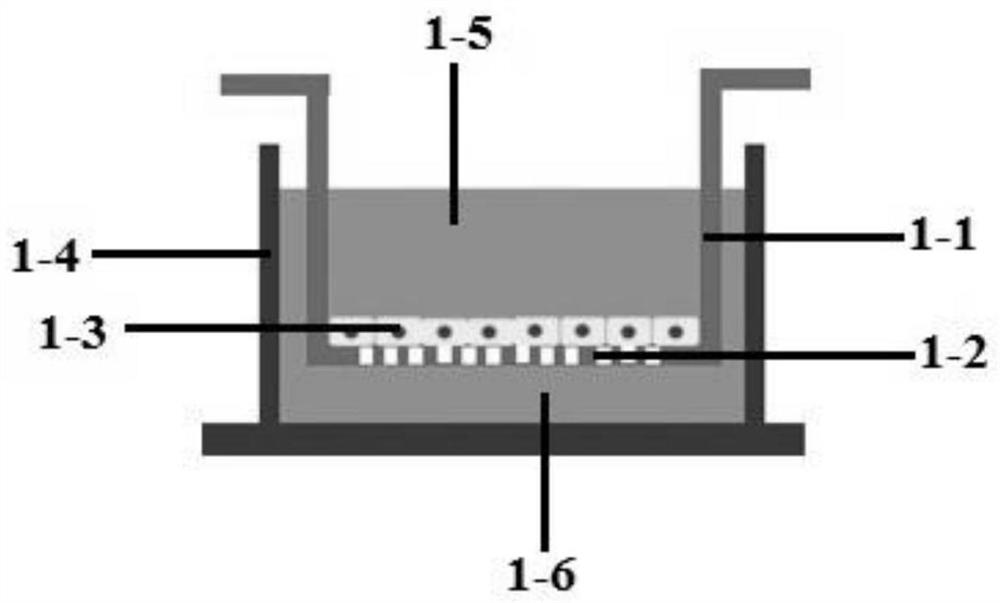
[0096] Embodiment 1: the establishment of BBB model
[0097]After ten two-week-old SD rats were killed by cervical dislocation, the brains were taken out in D-Hank's solution under ice bath conditions, the obtained cerebral cortex was cut into 1ml DMEM, and the cortex was added to 4ml 0.1% II Type II collagenase was thoroughly mixed with the cortex by hand shaking; put the mixture into a centrifuge tube and react in a water bath at 37°C for 1.5 hours. During this period, the centrifuge tube was shaken 6 times. Centrifuge the centrifuge tube at 1000r / min for 10min, discard the supernatant; add 20% BSA to the centrifuge tube to resuspend, centrifuge the solution at 4°C and 1000g for 20min, remove excess tissue, discard the supernatant, Add 2ml of 0.1% collagenase / dispase to the precipitate in the centrifuge tube, put it in a 37°C water bath for 1h, then centrifuge at 1000r / min for 8min at room temperature, discard the supernatant; add to the centrifuge tube Resuspend the pell...
Embodiment 2
[0098] Example 2: Evaluation of the BBB model
[0099] (1) 4h leakage test: add 2.5ml ECM to the upper tank of the obtained BBB model, add 1.5ml ECM to the lower tank, so that the upper tank and the lower tank have a liquid level difference > 0.5cm, put it in 37 ° C, 5% CO 2 After culturing in the incubator for 4 hours, observe whether the liquid level difference decreases. If the liquid level difference does not decrease, the cells have completely covered the membrane;
[0100] (2) TEER detection: After the 4h leak test is completed, use a pipette gun to level the liquid level difference, take the probe of the cell resistance meter (MillicellERS-2), and place the long head of the probe in the lower pool of the Tanswell 6-well plate, the probe Place the short head in the pool on a Tanswell 6-well plate, read the resistance value and record it, then put the probe into the blank well (that is, only the culture medium is not seeded with cells) read the resistance value and rec...
Embodiment 3
[0102] Example 3: Establishment and evaluation of an in vitro model of organophosphorus compound sarin central system poisoning and its role in evaluating Application of Activator in Pharmacological Effects
[0103] 0.07mg / ml AChE reagent, 10 -6 v / v sarin reagent and various reactivators (HI-6, bisphosphonate, pralidoxime, MMB-4 reagent) with a concentration of 0.03 mg / ml, among which, the above-mentioned several are existing reactivators agent;
[0104] (1) Establishment of an in vitro model of organophosphorus compound sarin central system poisoning:
[0105] First add 1.6ml of AChE to the lower pool of the BBB model obtained in Example 1, and then add 0.4ml of sarin reagent. After 3 minutes, the in vitro model I of sarin central system poisoning is established.
[0106] (2) In vitro model evaluation of sarin central system poisoning:
[0107] The Ellman method was used to detect the lower pool of model I, and its AChE inhibition rate was 78.61%. In the field, the ACh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com