Method for evaluating curative effect of compound glutamine formula on diarrhea-predominant irritable bowel syndromes
A technology of irritable bowel syndrome and glutamine, which is applied in the field of medicine, can solve the problems of dose usage analysis, inability to accurately evaluate the efficacy and advantages of compound glutamine prescriptions for diarrhea-predominant irritable bowel syndrome, and achieve cost Low cost, mature quarantine method and strong reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
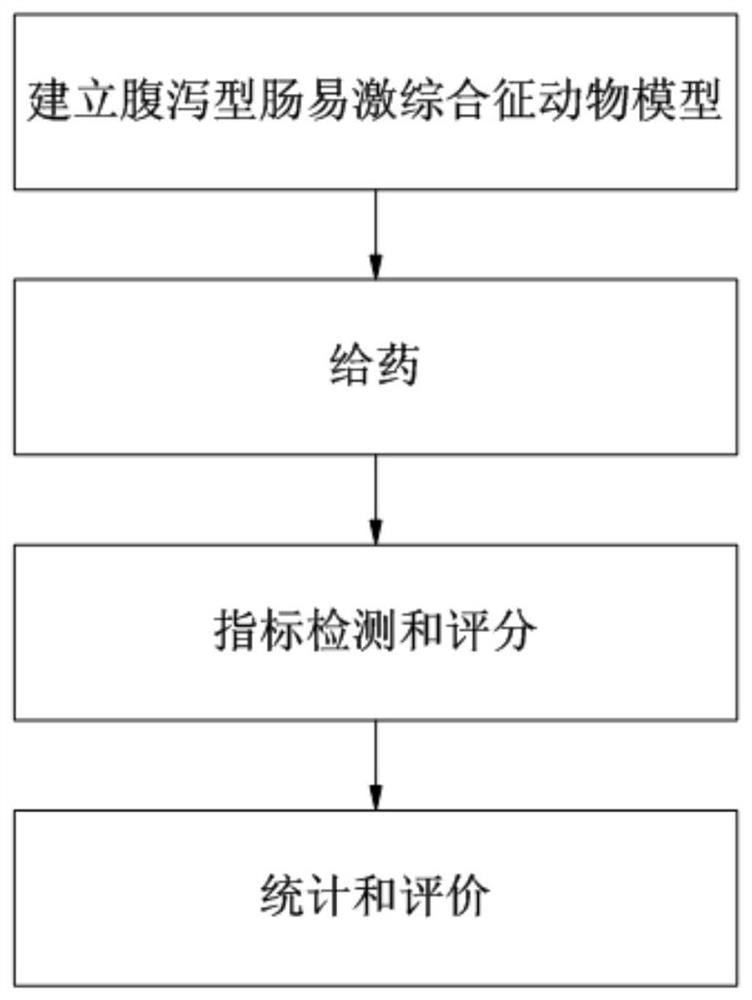
[0044] The invention provides a method for evaluating the curative effect of a compound glutamine prescription on diarrhea-predominant irritable bowel syndrome, which mainly includes establishment of an animal model of diarrhea-predominant irritable bowel syndrome, drug administration, index detection and scoring, statistics and evaluation. large processes, such as figure 1 shown. The process of the present invention will be further described below with reference to the accompanying drawings and embodiments, and the modes of the present invention include but not limited to the following embodiments.
[0045] The materials are as follows:
[0046] 1. Drugs and reagents
[0047] Compound glutamine enteric-coated capsules, specification: 0.2g content / capsule, containing 0.12g L-glutamine and 0.08g Sijunzi decoction, batch number: 190407.
[0048]Compound glutamine gastric-soluble capsules, specification: 0.2g content / capsule, containing 0.12g L-glutamine and 0.08g Sijunzi deco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com