Synthetic method for continuous saponification and decarboxylation of 4-methyl-5-ethoxyoxazole
A technology of ethoxyoxazole and ethyl ethoxyoxazole, applied in the field of compound synthesis, can solve the problems of low yield of 4-methyl-5-ethoxyoxazole, long reaction time and the like, and achieves Avoid prolonged contact, improve reaction yield and reduce energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
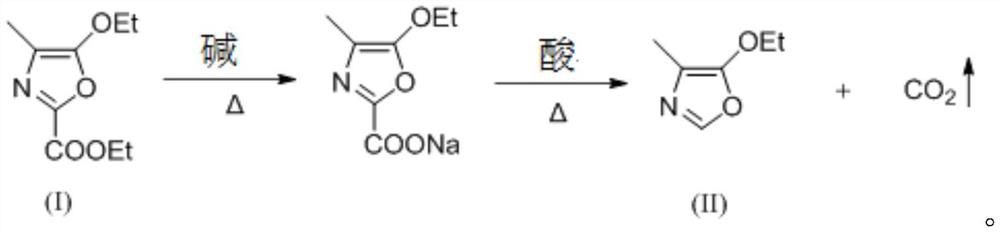
[0032] The synthetic method of the continuous saponification decarboxylation of 4-methyl-5-ethoxy oxazole provided by the invention comprises:
[0033] S1. The organic solution containing ethyl 4-methyl-5-ethoxyoxazole acid is premixed with alkali and then continuously pumped into the tubular reactor I for saponification reaction to obtain a saponification solution;
[0034] S2. The saponification liquid is premixed with acid and alcohol and then continuously pumped into the tubular reactor II for decarboxylation reaction. The obtained decarboxylation liquid is discharged from the outlet of the tubular reactor II to obtain 4-methyl-5- Ethoxyxazole.
[0035] The present invention has no particular limitation on the specific types of tubular reactor I and tubular reactor II, which can be various existing tubular reactors that can realize continuous synthesis and reduce back-mixing of reactants. The length-to-diameter ratio of the two Preferably, each independently is (50-200):1...
Embodiment 1
[0046] S1, respectively configure 1mol / L 4-methyl-5-ethoxy oxazole ethyl ester toluene solution and 20% w / w sodium hydroxide aqueous solution, these two materials are 1 according to unit time volume ratio: The ratio of 0.25 is fully mixed with a mixer and preheated to 50°C, then sent to the tubular reactor I (straight tubular reactor) for reaction, the temperature of the reactor is controlled at 60°C, and the residence time is 5 minutes to obtain a saponified liquid ;
[0047] S2. Fully mix the saponification solution with concentrated hydrochloric acid (concentration is 36% to 38%, the same below) and ethanol according to the volume ratio of 1:0.12:0.25 per unit time and preheat to 60°C, then send it into the tube Type reactor II (straight tube reactor) reaction, control the temperature of the reactor at 65°C, and the residence time is 10 minutes, the decarboxylation liquid is discharged from the outlet of the tube reactor II, separate layers, the water layer is extracted onc...
Embodiment 2
[0049] Synthesize 4-methyl-5-ethoxy oxazole according to the method of Example 1, the difference is that the organic solvent for dissolving 4-methyl-5-ethoxy oxazole ethyl ester is replaced by chloroform, The preheating temperature of tubular reactor II was changed to 50° C., the decarboxylation reaction temperature was changed to 55° C., and the decarboxylation residence time was changed to 20 minutes. The remainder was the same as in Example 1 to obtain 4-methyl-5-ethoxy oxazole, Its content is 99.10%, and the yield is 97.83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com