A kind of synthetic method of 4-methyl-5-ethoxyoxazole continuous saponification decarboxylation
A technology of ethoxyoxazole and ethyl ethoxyoxazole, applied in the field of compound synthesis, can solve the problems of low yield of 4-methyl-5-ethoxyoxazole, long reaction time and the like, and achieves The effect of avoiding prolonged contact, improving the reaction yield and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
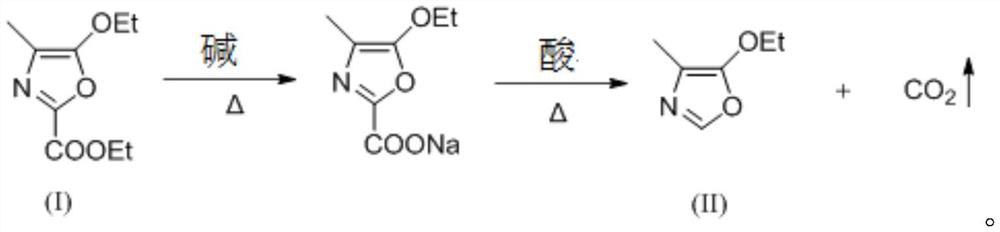
[0032] The synthetic method of 4-methyl-5-ethoxyoxazole continuous saponification decarboxylation provided by the invention comprises:
[0033] S1, the organic solution containing 4-methyl-5-ethoxyethyl oxazolate is continuously pumped into tubular reactor I after premixing with alkali to carry out saponification reaction to obtain saponification solution;
[0034] S2. After the saponification liquid is premixed with acid and alcohol, it is continuously pumped into tubular reactor II for decarboxylation reaction, the obtained decarboxylation liquid is discharged from the outlet of tubular reactor II, and 4-methyl-5- Ethoxyxazole.
[0035] There is no particular limitation on the specific types of the tubular reactor I and the tubular reactor II in the present invention, which can be various existing tubular reactors that can realize continuous synthesis and reduce back-mixing of reactants. Preferably, each independently is (50 to 200):1. Specifically, the tubular reactor I a...
Embodiment 1
[0046] S1, configure the 4-methyl-5-ethoxy oxazole acid ethyl ester toluene solution of 1mol / L respectively and the sodium hydroxide aqueous solution of 20%w / w, these two materials are 1 according to the volume ratio per unit time: After the ratio of 0.25 was fully mixed by the mixer and preheated to 50°C, it was sent to the tubular reactor I (straight-tube reactor) for reaction, the temperature of the reactor was controlled to be 60°C, and the residence time was 5 minutes to obtain a saponified solution. ;
[0047] S2, fully mix the saponification solution with concentrated hydrochloric acid (concentration is 36%~38%, the same below) and ethanol in a ratio of 1:0.12:0.25 according to the volume ratio per unit time and preheat to 60°C, then feed into the pipe Type reactor II (straight-tube reactor) reaction, control the temperature of the reactor to be 65 ℃, the residence time is 10 minutes, the decarboxylation liquid is discharged from the outlet of the tubular reactor II, an...
Embodiment 2
[0049] Synthesize 4-methyl-5-ethoxyoxazole according to the method of Example 1, the difference is that the organic solvent for dissolving ethyl 4-methyl-5-ethoxyoxazole is replaced with chloroform, The preheating temperature of the tubular reactor II was changed to 50°C, the decarboxylation reaction temperature was changed to 55°C, and the decarboxylation residence time was changed to 20 minutes, the same as in Example 1, to obtain 4-methyl-5-ethoxyoxazole, Its content was 99.10%, and the yield was 97.83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com