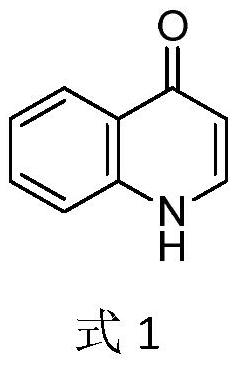
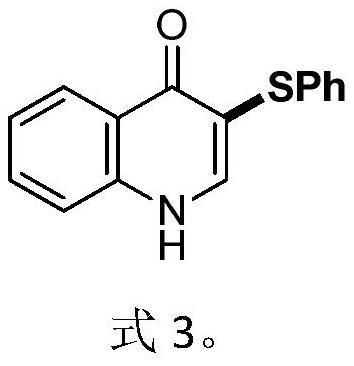
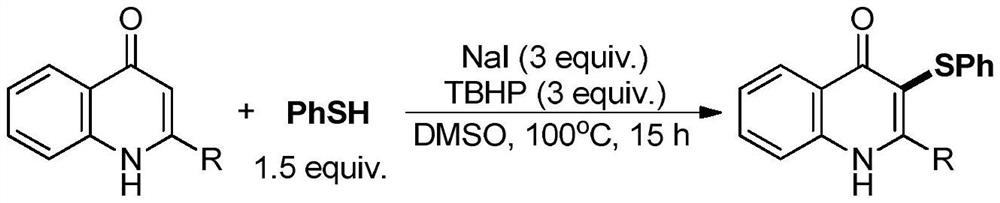Electrochemical synthesis method of 3-phenylthio quinolinone
A technology of phenylthioquinolinone and synthesis method, which is applied to electrodes, electrolysis process, electrolysis components, etc., can solve the problems of difficult separation and purification, high reaction cost, excess, etc., and achieves easy product separation and purification, and reaction selectivity. High and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] The specific operation steps are: in a 25mL three-neck round bottom flask, add 4-quinolinone (5mmol), thiophenol (5mmol), sodium iodide (0.5mmol), hexafluoroisopropanol (10mL), 15mm× A 15mm×4mm foam iron electrode is used as an anode, and a 15mm×15mm×4mm foam copper electrode is used as a cathode. The resulting mixed solution was stirred and reacted under 16mA direct current at room temperature for 20 hours. After the reaction was completed, 10mL of water was added to precipitate the product, filtered and dried to obtain the pure product.
[0034] 94%, 3-(phenylthio)quinolin-4(1H)-one
[0035] 1 H NMR (400MHz, CDCl 3 )δ7.08–7.14(m,3H),7.23(t,J=7.8Hz,2H),7.41(t,J=7.8Hz,1H),7.62(d,J=7.8Hz,1H),7.68– 7.72(m, 1H), 8.12(d, J=7.8Hz, 1H), 8.39(d, J=6.0Hz, 1H), 12.32(d, J=5.2Hz, 1H)
[0036] 13 C NMR (100MHz, CDCl 3 ) δ 109.4, 118.5, 124.0, 125.0, 125.1, 125.4, 126.4, 128.9, 132.1, 137.6, 139.8, 145.4, 174.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com