Jak1 pathway inhibitors for the treatment of cytokine-related disorders
A technology of cytokines and inhibitors, applied in the field of JAK1 pathway inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0156] Example A: In Vitro JAK Kinase Assay
[0157] JAK1 pathway inhibitors useful in the treatment of cytokine-related diseases or disorders are tested for their inhibitory activity on JAK targets according to the following in vitro assay described in Park et al., Analytical Biochemistry 1999, 269, 94-104. The catalytic domains of human JAK1 (a.a.837-1142), JAK2 (a.a.828-1132) and JAK3 (a.a.781-1124) with an N-terminal His tag were expressed in insect cells using baculovirus and purified. The catalytic activity of JAK1, JAK2 or JAK3 was determined by measuring the phosphorylation of biotinylated peptides. Phosphorylated peptides were detected by homogeneous time-resolved fluorescence (HTRF). Compound activity against each kinase was measured in 40 microliter reactions containing enzyme, ATP and 500 nM peptide in 50 mM Tris (pH 7.8) buffer with 100 mM NaCl, 5 mM DTT and 0.1 mg / mL (0.01%) BSA. IC 50 . For 1mM IC 50The ATP concentration in the reaction was measured to be 1...
Embodiment B
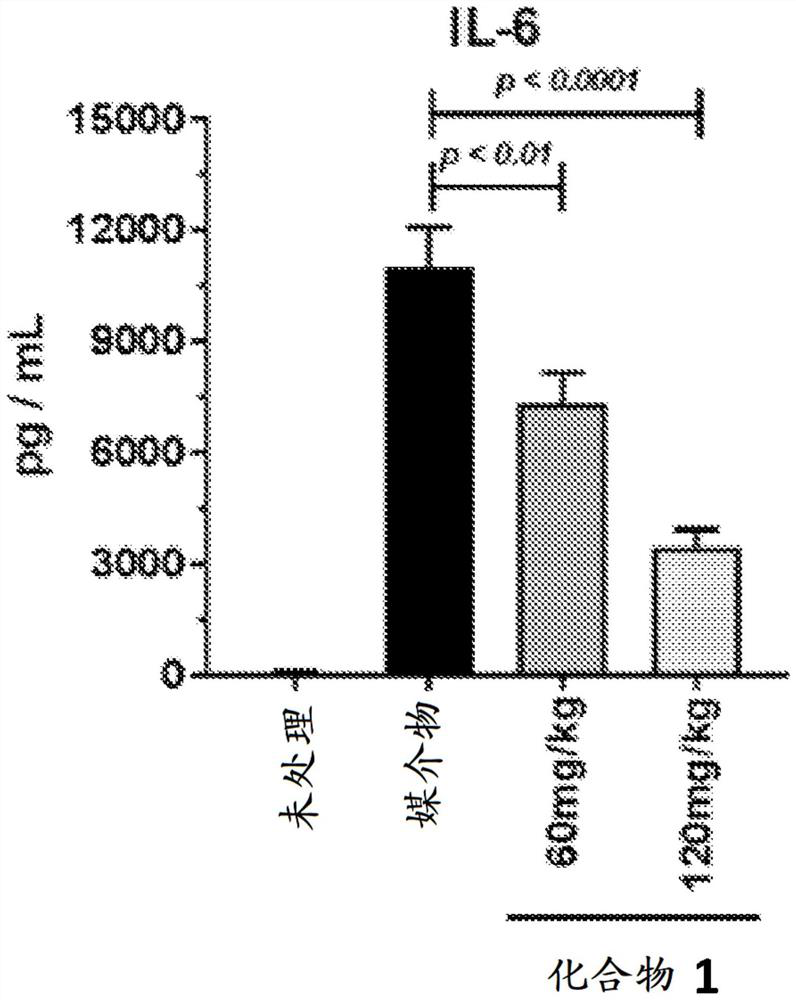
[0158] Example B: Cytokine Release Syndrome Induced by Anti-CD3 Antibody in BALB / c Mice
[0159] The efficacy of JAK1 pathway inhibitors against CRS can be tested according to the in vivo assay described in Ferran, C. et al. Clin. Exp. Immunol. 1991, 86, 537-543. Specifically, this study tested the ability of compounds to reduce or ameliorate anti-CD3 antibody-induced cytokine release syndrome (CRS) in BALB / c mice. Antibody clone 145-2C11 is an immunoglobulin G (IgG) hamster MoAb specific for the epsilon chain of the CD3 murine molecule (Léo, O. et al., Proc. Natl. Acad. Sci. USA, 1987, 34, 1374 ). Treatment with 145-2C11 induces high-affinity IL-2 receptors on the surface of splenic T cells and elicits e.g. tumor necrosis factor (TNF-α), IL-2, IL-3, IL-6 and interferon-γ (IFN -γ) release of some cytokines (Ferran et al., Eur. J. Immunol. 1990, 20, 509-515 and Algre, M. et al., Eur. J. Immunol., 1990, 707). The release of these cytokines causes behavioral changes in the ani...
Embodiment C
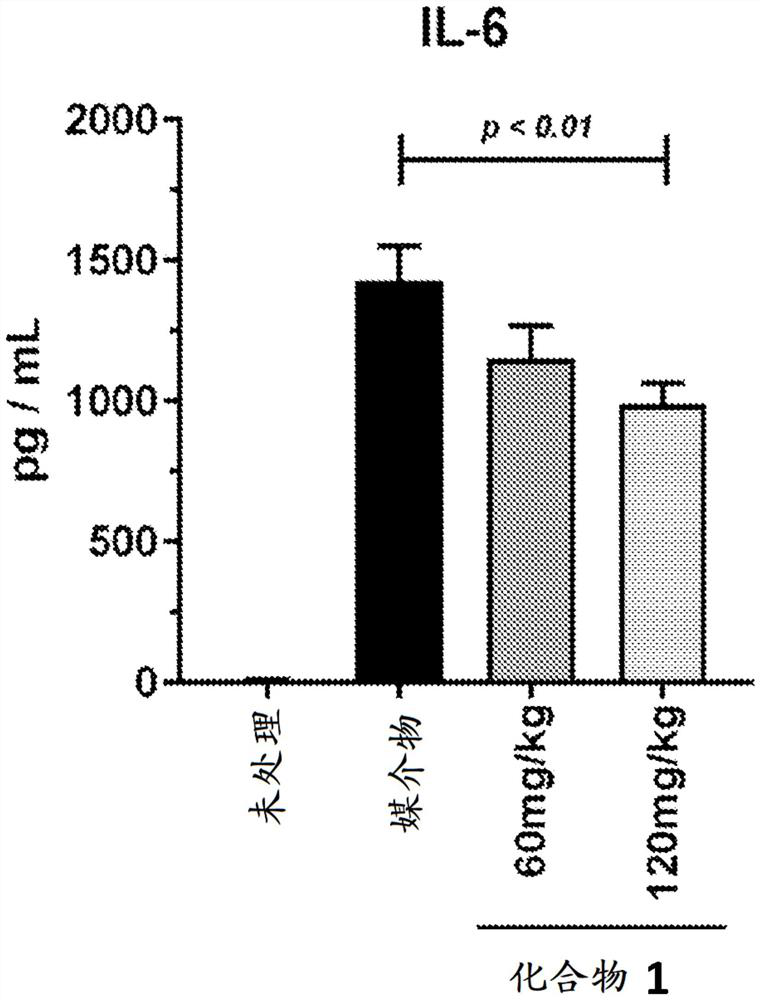
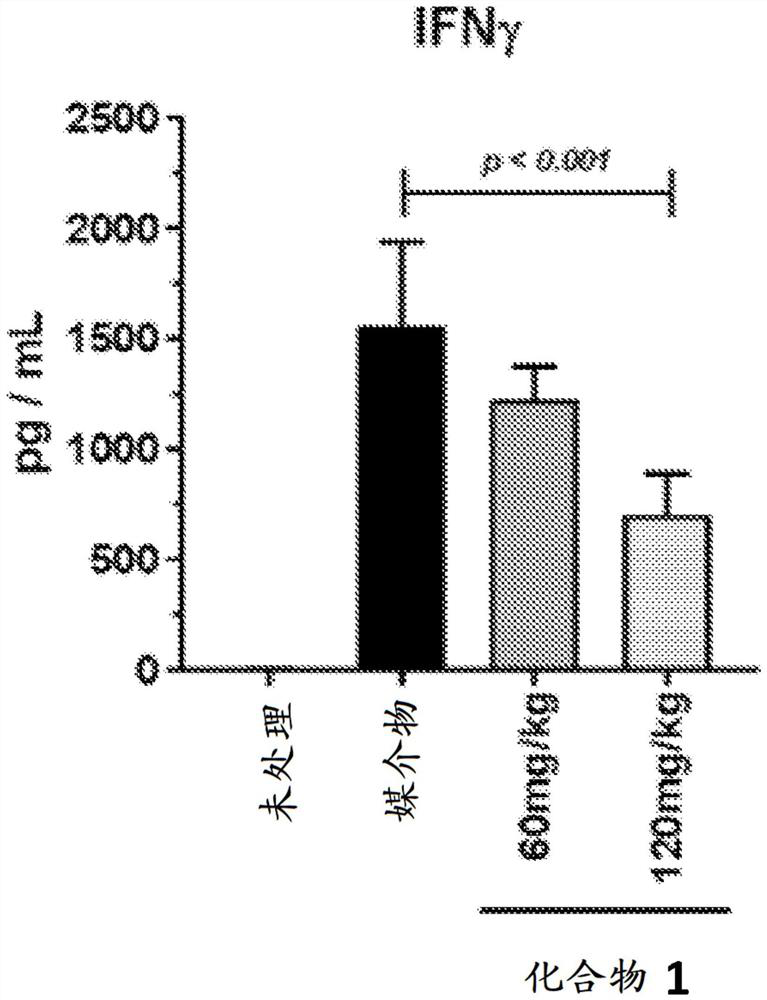
[0183] Example C: Concanavalin A-Induced Cytokine Release Syndrome
[0184] Concanavalin A (Con A) is a selective T lymphocyte mitogen that causes widespread inflammatory cytokine release and proliferation of CD4 and CD8 T cells. Injection of Con-A has been shown in the literature to induce cytokine release syndrome and was used as a model to test the efficacy of cytokine release syndrome therapy (Gantner, F. et al. Hepatology, 1995, 21, 190-198). The mitogen response depends on the expression of T cell receptors. Animals exhibit behavioral changes such as fever, malaise, hypotension, hypoxia, capillary leak, and potential multi-organ toxicity.
[0185] A.Materials and methods
[0186]
[0187] B. Experimental design
[0188] In detail, this study tests the ability of selective JAK1 inhibitors (such as compound 1 of Table 1) to reduce or ameliorate Con A-induced cytokine release syndrome (CRS) in BALB / c mice. A total of forty (40) BALB / c mice were used in this one d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com