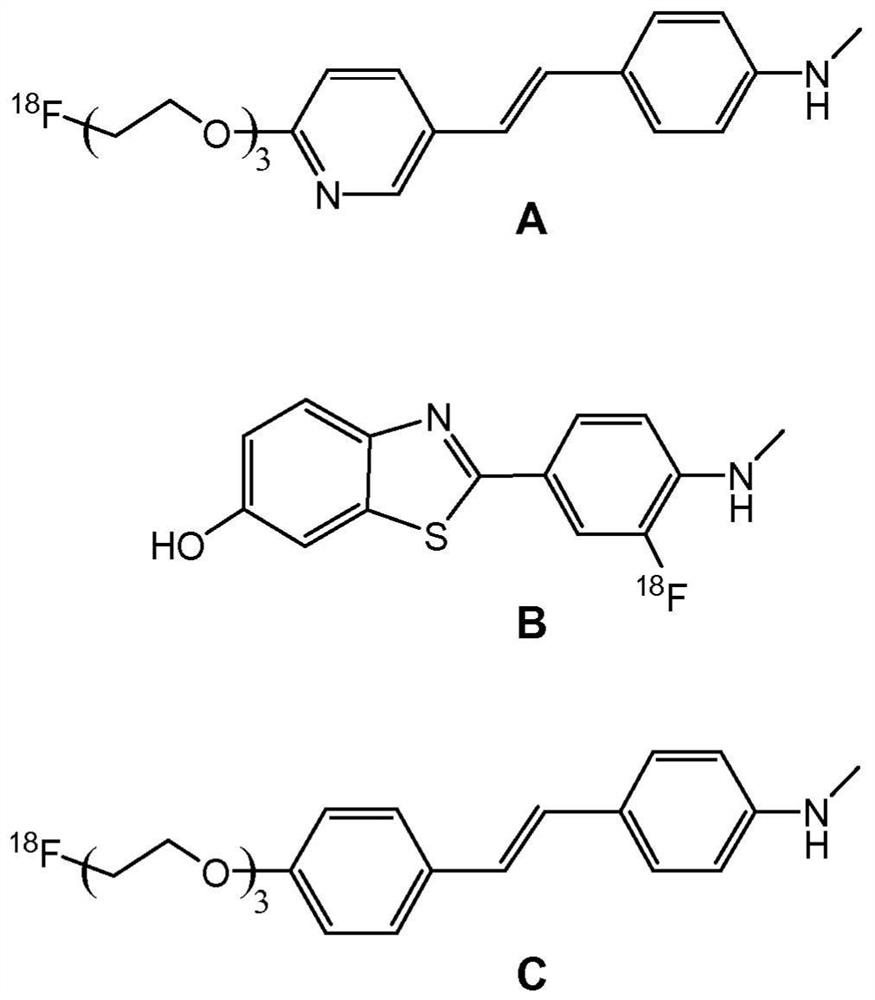
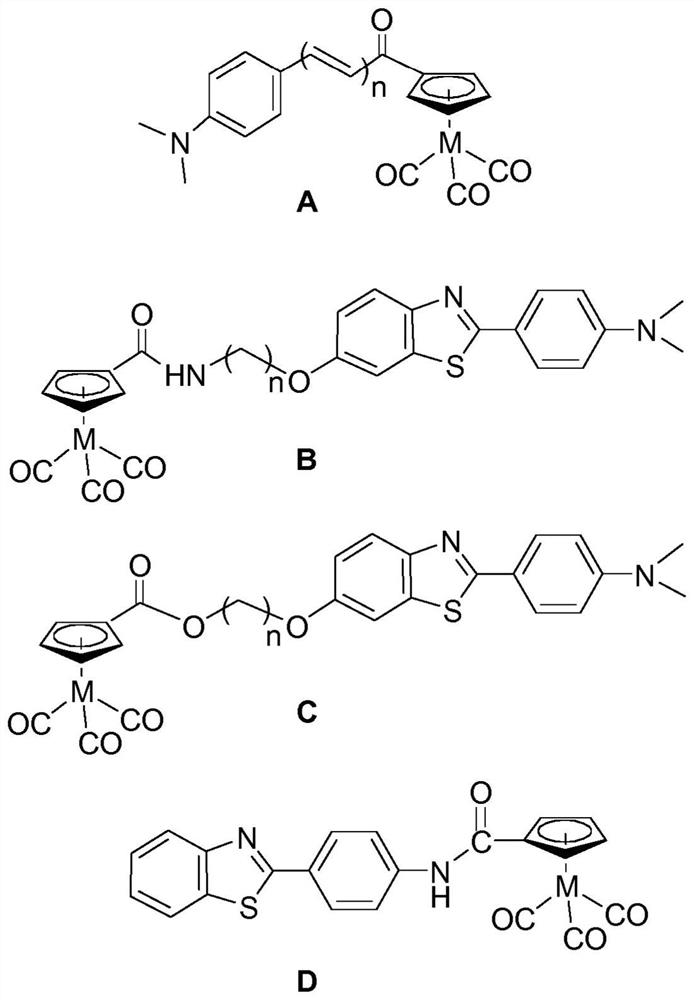
Tricarbonyl complexes of transition metals with benzo-heterocyclic derivatives of the cyclopentadienyl anion
A transition metal, tricarbonylcyclopentadiene technology, applied in the field of therapeutic applications, can solve the problem of low cure rate and achieve the effect of high blood-brain barrier permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 9
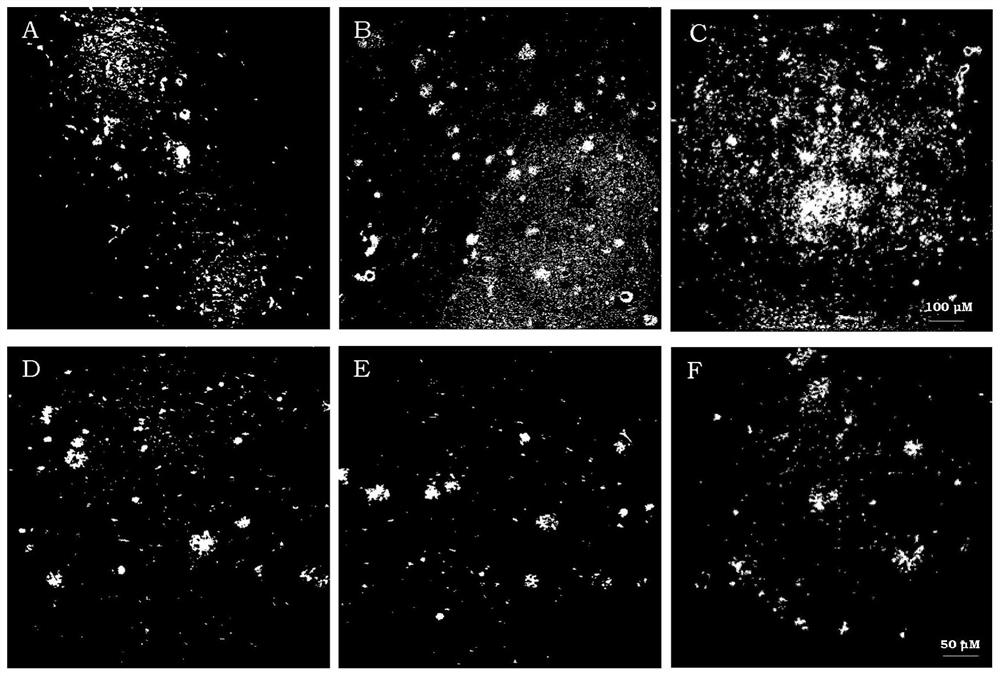
[0041] The use of the complex in Alzheimer's disease-related challenges, including the staining of amyloid plaques in brain tissue using the complex Re-2-Re-5 (Example 10), the complex 99m Tc-2-99m Determination of the affinity constant for the binding of Tc-4 to amyloid fibrils (Example 11), inhibition of amyloid fibril-induced toxicity in primary neuronal cells using the complex Re-2, Re-3 (Example 12), and the determination of the antioxidant activity of complexes Re-2 and Re-3 against ROS (Example 13).
Embodiment 1
[0044] Synthesis of Ferrocenyl-Benzothiazole (Fe-2)
[0045]
[0046] Ferroceneformaldehyde (1.1 g, 5 mmol) and 2-aminothiophenol (600 μL, 5.6 mmol, 1.1 eq) in ethanol (40 mL) were stirred at reflux for 16 hours. The reaction was brought back to r.t and a reddish brown solid precipitated. The final product was obtained by flash column chromatography of the precipitate eluting with hexane:ethyl acetate (98:2 to 96:4). Yield: 92%. NMR (DMSO-d 6 ,ppm): 1 Η(500MHz)8.03,7.92,7.47,7.39,5.02,4.60,4.16; 13 C(126MHz)153.43,126.24,124.52,121.96,121.80,70.82,70.13,68.50.MS(ESI)m / z:[M+H] + Calculated value C 17 h 14 NS 56 Fe, 320.0188; measured value, 320.0188.
Embodiment 2
[0048] Synthesis of Ferrocenyl-Benzimidazole (Fe-3)
[0049]
[0050] A mixture of ferroceneformaldehyde (0.76 g, 4 mmol) and sodium bisulfite (1.44 g, 1.4 mmol) was dissolved in a mixture of 20 mL of absolute ethanol and water (1:1). The reaction mixture was refluxed for 1 hour. o-Phenylenediamine (0.4 g, 4 mmol) was added at room temperature and the mixture was refluxed for another 1 hour. Then cool to room temperature and finally refrigerate. The formed precipitate was filtered and washed successively with ice cold water and cold absolute ethanol. The pure product was isolated by flash column chromatography of the precipitate eluting with dichloromethane:ethyl acetate (96:4). Yield: 62%. NMR (DMSO-d 6 ,ppm): 1 Η(500MHz) 12.35, 7.54, 7.44, 7.13, 5.04, 4.47, 4.10; 13 C NMR (126 MHz) 152.92, 120.86, 117.50, 110.15, 69.31, 68.91, 66.90.
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com