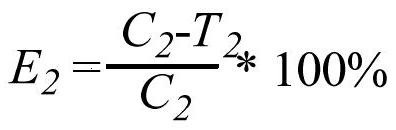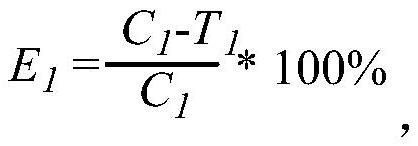Thiotriazinone isoxazoline compound, preparation method and application thereof, protoporphyrinogen oxidase inhibitor and herbicide
A technology containing thiotriazinone and isoxazoline, which is applied in the field of pesticides, can solve the problems that cannot be applied to the growth process of crops, and achieve good herbicidal activity, high crop safety, and the effect of weed removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
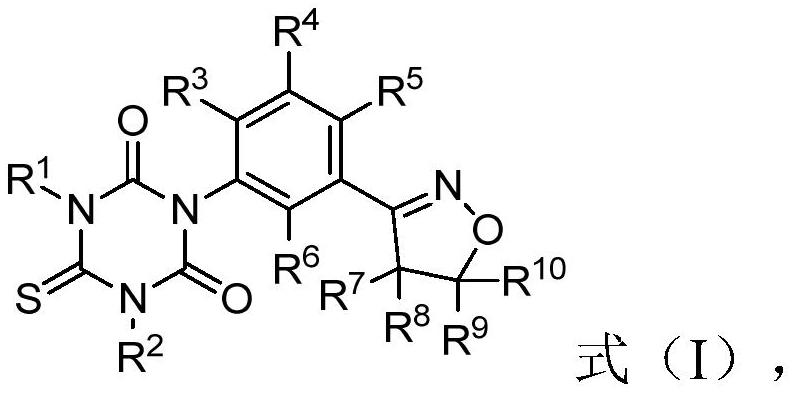
[0047] for R 5 , preferably, R 5 is selected from H, halogen or -CN; more preferably, R 5 is selected from halogen or -CN. According to a specific embodiment of the present invention, R 5 selected from -Cl or -CN.
[0048] for R 6 , preferably, R 6 is selected from H, halogen or -CN. According to a specific embodiment of the present invention, R 6 for H.
[0049] for R 7 , preferably, R 7 Choose from H, C 1 -C 6 Alkyl or C 1 -C 8 Haloalkyl; more preferably, R 7 selected from H, -CH 3 , -CH 2 CH 3 or halomethyl. According to a specific embodiment of the present invention, R 7 selected from H or -CH 3 .
[0050] for R 8 , preferably, R 8 Choose from H, C 1 -C 6 Alkyl, C 1 -C 8 Haloalkyl; more preferably, R 8 selected from H, -CH 3 , -CH 2 CH 3 or halomethyl. According to a specific embodiment of the present invention, R 8 selected from H or -CH 3 .
[0051] for R 9 , preferably, R 9 selected from H, -CN, C 1 -C 6 Alkyl, C 1 -C 6 Haloalkyl...
Embodiment approach
[0104] According to a preferred embodiment of the present invention, the condensation reaction comprises the following steps:
[0105] The first step: in the presence of the third solvent, the R 10 A compound represented by the formula (I) of -CO-OH is subjected to an acyl chloride reaction with an acyl chloride reagent to obtain an acyl chloride intermediate;
[0106] Second step: in the presence of the fourth solvent and the third base, the acid chloride intermediate and Structures of compounds undergo chemical reactions.
[0107] In the first step, preferably, the R 10 The molar ratio of the compound represented by the formula (I) which is -CO-OH to the acid chloride reagent is 1:(1.05-3).
[0108] In the first step, preferably, the process of the acid chloride reaction includes the following two stages, wherein stage 1-1 is: at 0-5°C, the third solvent, R 10The compound represented by the formula (I) which is -CO-OH and the acid chloride reagent are mixed and contacte...
Embodiment 3
[0139] Embodiment 3 --- the preparation of compound 3
[0140] Carry out according to the reaction process shown in following formula IV,
[0141]
[0142] This embodiment is used to prepare compound 3 shown in Table 1, as recorded in Table 1, in the above-mentioned formula (IX)-formula (I), R 1 =CH 3 , R 2 =CH 3 , R 3 = F, R 4 = H, R 5 = Cl, R 6 = H, R 7 = H, R 8 = H, R 9 =CH 3 , R 10 =CO-OC 2 h 5 .
[0143] In order to be more intuitive, the above formula IV is specifically rewritten as the process of preparing compound 3, as shown in the following formula V.
[0144]
[0145] The specific process includes:
[0146] 1) Preparation of Compound IV
[0147] Dissolve 0.206 mol of the compound represented by formula (IX) in 200 mL of ethanol, lower it to 0° C., add dropwise 0.25 mol of an aqueous solution of hydroxylamine hydrochloride under stirring, and then rise to room temperature and stir to react. After 2 hours, TLC detected that the reaction was com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com