Triazolopyrimidinylsulfonamide compound, composition containing compound, and application of compound
An azolopyrimidine sulfonamide and compound technology, applied in the field of triazolopyrimidine sulfonamide compounds, can solve problems such as hindering amino acid synthesis, plant metabolism disorder, plant growth damage, etc., achieve high growth inhibitory activity, low preparation cost, The effect of fewer synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] According to the second aspect of the present invention, there is also provided a preparation method of the triazolopyrimidine sulfonamide compound represented by formula (I), which comprises the following steps:
[0054] step 1) , Preparation of ethoxycarbonyl isothiocyanate: using ethyl acetate as a solvent, potassium thiocyanate and ethyl chloroformate are substituted to obtain a mixture of ethoxycarbonyl isothiocyanate and ethyl acetate;
[0055] Step (2) Preparation of N-(substituted pyrimidinyl)-N′-ethoxycarbonylthiourea: Add 2-amino-substituted pyrimidine to the mixture of ethoxycarbonyl isothiocyanate and ethyl acetate prepared in step (1) Or 4-amino substituted pyrimidine, and carry out a substitution reaction to obtain the product;
[0056] Step (3) Preparation of 2-amino-triazolo-substituted pyrimidine: using ethanol as a solvent, under alkaline conditions, hydroxylamine hydrochloride and the N-(substituted pyrimidinyl)-N′-ethoxycarbonylthiourea prepared in step ...
Embodiment 1
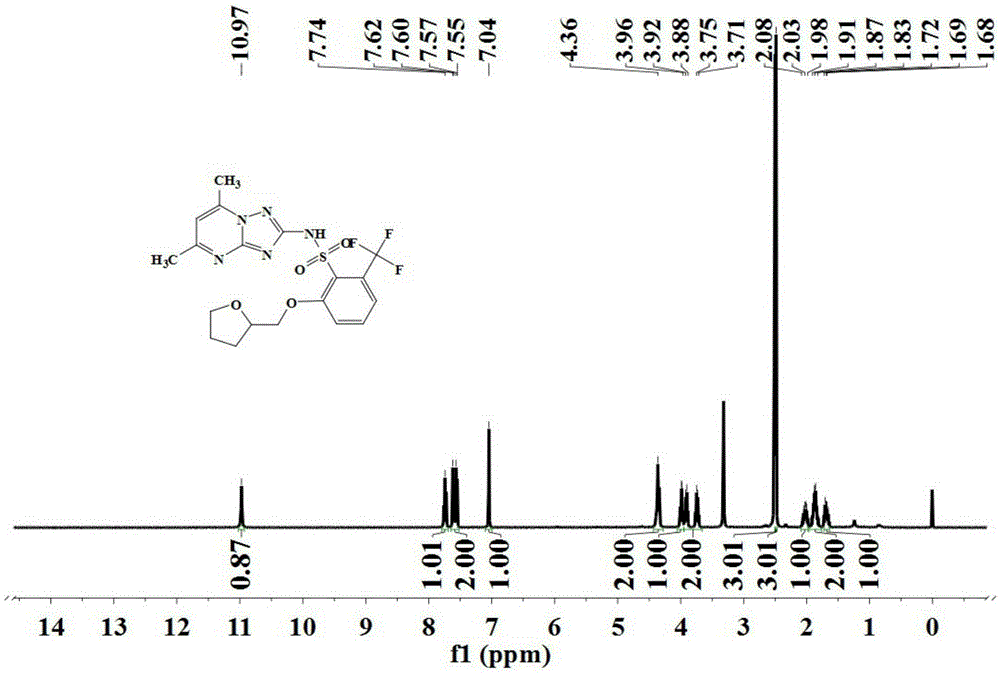
[0071] The triazolopyrimidine sulfonamide compound represented by formula (III) is prepared according to the method described in steps (1) to (7).
[0072]
[0073] step 1) , Preparation of ethoxycarbonyl isothiocyanate
[0074] In a 1000mL four-necked flask equipped with a stirrer, thermometer, reflux condenser, and dropping funnel, add 600mL ethyl acetate, add 97.2g (1mol) potassium thiocyanate under stirring, heat to 40℃, and add 106.3g dropwise (0.98mol) Ethyl chloroformate was added dropwise at a temperature lower than 45°C. After the addition, the temperature was controlled at 40°C and kept for 2h. When the reaction of ethyl chloroformate was completed, it was cooled to room temperature and filtered to remove the potassium salt. The mixture of ethoxycarbonyl isothiocyanate and ethyl acetate is ready for use.
[0075] Step (2) , Preparation of N-(4,6-Dimethoxypyrimidin-2-yl)-N′-ethoxycarbonylthiourea
[0076] Add 124.1g (0.8mol) of 2-amino-4,6-dimethoxypyrimidine to the reacti...
Embodiment 2
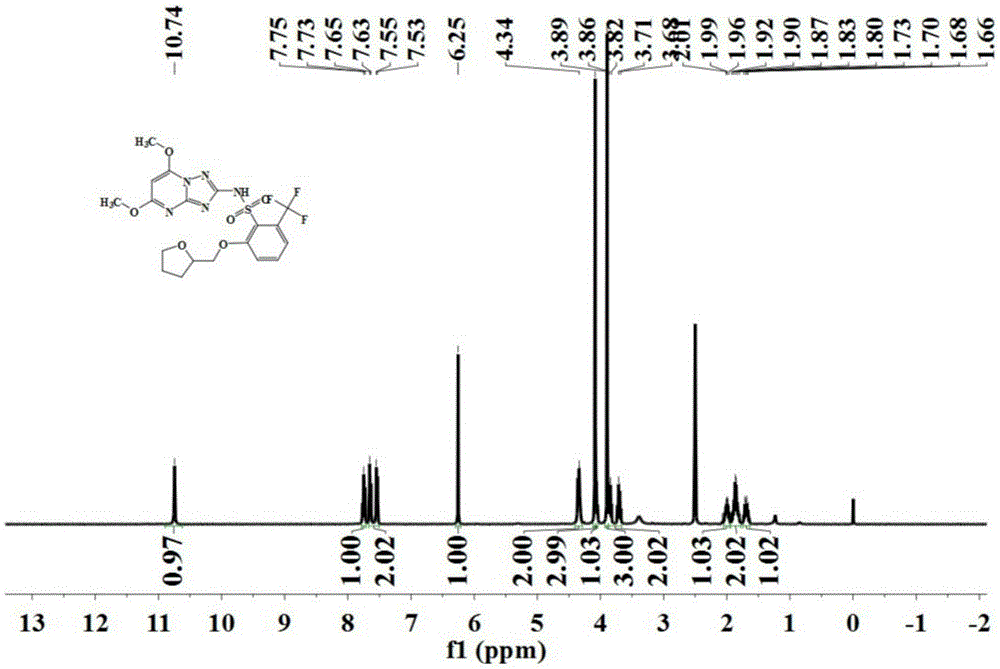
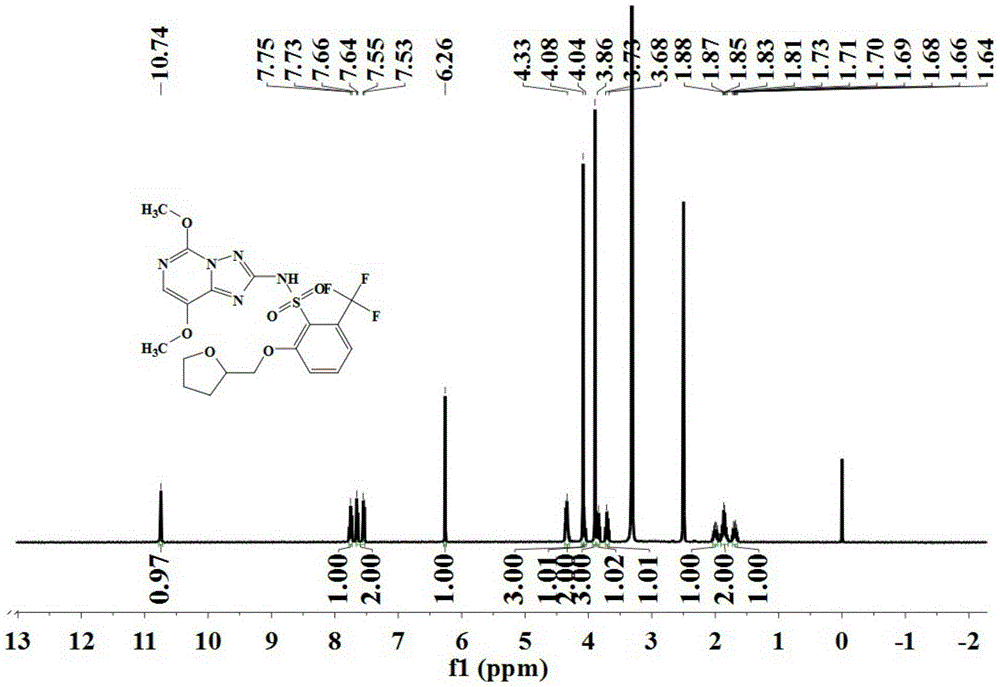
[0088] The preparation was carried out according to the method similar to that of Example 1, except that the 2-amino-4,6-dimethoxypyrimidine in step (2) was replaced with the same amount of 2,5-dimethoxy-4 -Aminopyrimidine to obtain the triazolopyrimidine sulfonamide compound represented by formula (IV) with a purity of 96.1%, and its hydrogen nuclear magnetic resonance spectrum as shown in figure 2 Shown.
[0089]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com