Application of a condensation product of a pillar aromatic hydrocarbon and a crown ether hybrid macrocycle as an adhesive
A technology of pillar aromatic hydrocarbons and condensates, which is applied in the application field of condensates as adhesives, which can solve problems such as failure and viscosity reduction, and achieve the effects of fast hardening speed, no change in adhesion ability, and simple adhesion process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The compound provided by this embodiment has the molecular structural formula as follows:
[0037]
[0038] In a 100-ml round-bottomed flask, drop 2.36 g of benzo-21-crown-7-carboxylic acid, 0.52 g of decaamine[5]arene and 1.44 g of 4-dimethylaminopyridine, dissolve in 50 ml of dichloromethane, After stirring in an ice bath for ten minutes, 4.54 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride was added and reacted at room temperature for 6 days. After adding 2 ml of 10% hydrochloric acid and stirring for 5 minutes, the layers were separated. The lower organic layer was washed with water and spin-dried to obtain a crude product. The crude product was separated and purified by a silica gel column to obtain 1.28 g of the product with a yield of 45%.
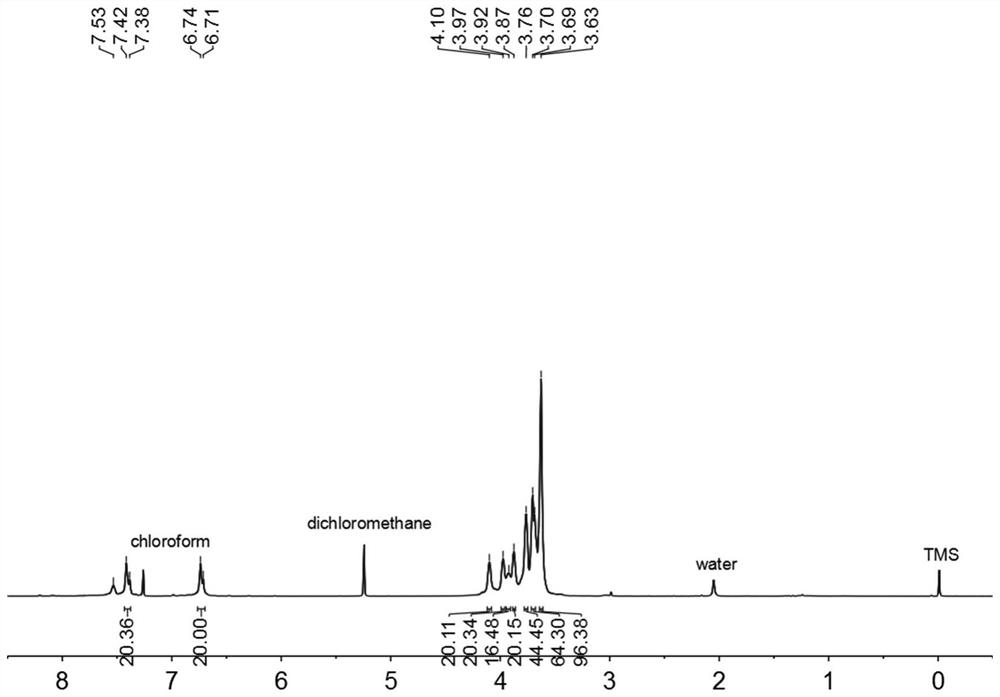
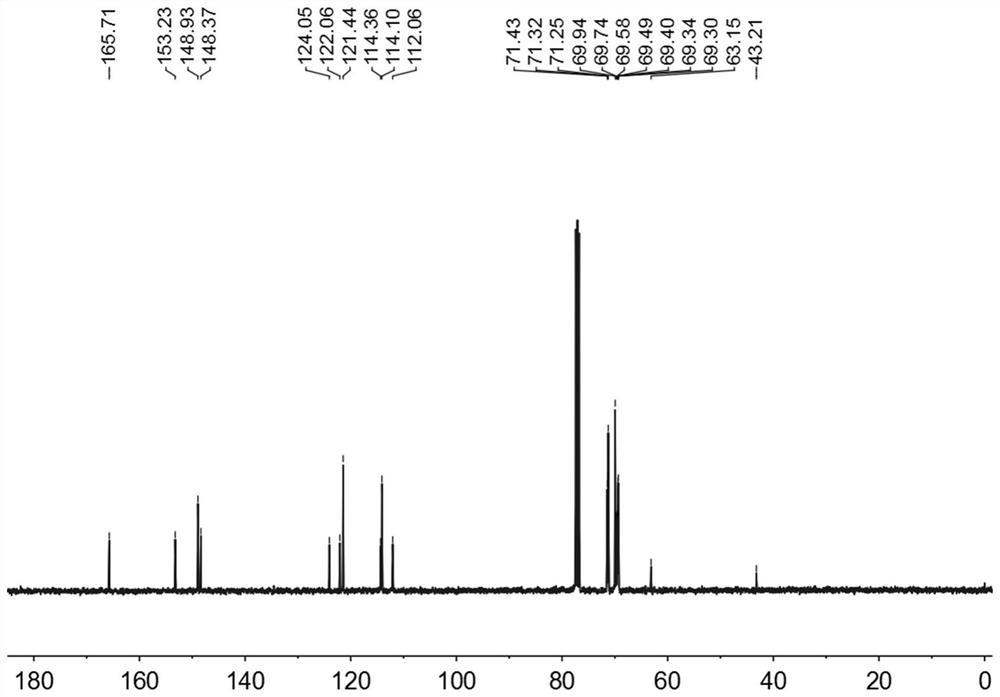
[0039] The hydrogen spectrum and carbon spectrum data are as follows:
[0040] 1 H NMR (400MHz, CDCl 3 ,room temperature)δ7.40(d,J=16.0Hz,20H),6.73(d,J=12.0Hz,20H),4.10(s,20H),3.97(s,20H),3.92(s,16H) ,...
Embodiment 2
[0043] The compound provided by this embodiment has the molecular structural formula as follows:
[0044]
[0045] In a 100-ml round-bottom flask, put 2.59 g of bisbenzo-24-crown-8-carboxylic acid, 0.48 g of decaamine[5]arene and 1.32 g of 4-dimethylaminopyridine, and dissolve with 50 ml of dichloromethane After stirring in an ice bath for ten minutes, 4.17 g of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride was added and reacted at room temperature for 6 days. After the reaction was completed, 2 ml of 10% hydrochloric acid was added and stirred for 5 minutes before separation. The lower organic layer was washed with water and spin-dried to obtain a crude product, which was separated and purified by a silica gel column to obtain 1.72 g of product with a yield of 55%.
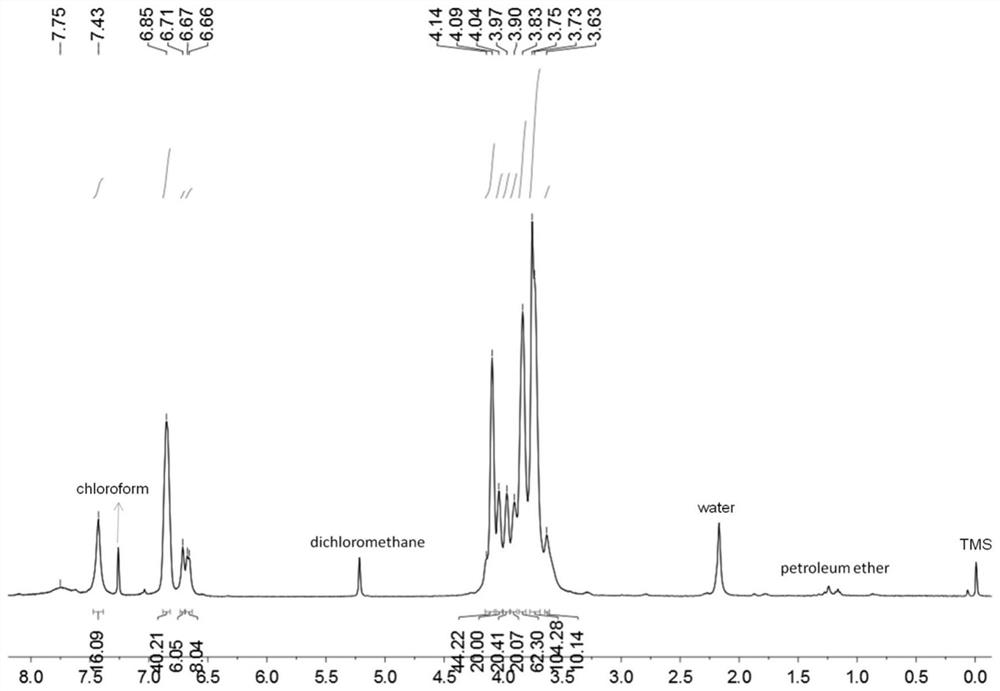
[0046] The hydrogen spectrum and carbon spectrum data are as follows:
[0047] 1 H NMR (400MHz, CDCl 3,room temperature)δ7.43(s,16H),6.85(s,40H),6.71(s,6H),6.67(d,J=8.0Hz,8H),4.14–4.09(m,44H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com