Biological membrane material adopting cell transplantation as carrier
A technology of cell transplantation and biofilm, applied in the direction of unknown raw materials, prostheses, medical preparations with non-active ingredients, etc., can solve the problems of greater repellency and achieve the effect of improving affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Raw material preparation:
[0038] 1) Full-term normal delivery of human fetal amniotic membrane,
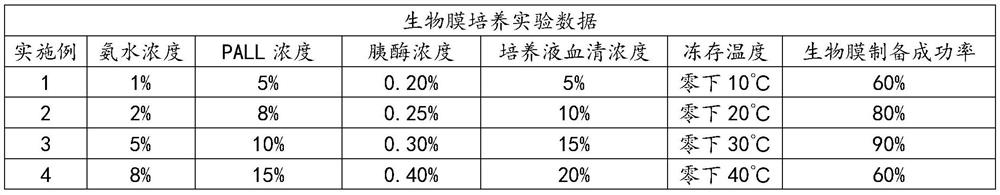
[0039] 2) 1% concentration of ammonia water,
[0040] 3) Antibiotics (using penicillin, clarithromycin, clindamycin),
[0041] 4) balanced salt solution (0.9% NACL solution),
[0042] 5) 0.2% trypsin solution,
[0043] 6) 5% concentration of PLLA solution,
[0044] 11) DMEM culture fluid containing 5% serum,
[0045] 12) Co60 radioactive source;
[0046] 2. Select the placenta within 30 minutes after delivery of the first child of healthy puerpera without infectious diseases and systemic diseases;
[0047] 3. Bluntly separate the amniotic membrane from the placenta under sterile conditions;
[0048] 4. Place the amniotic membrane in balanced salt solution for injection containing penicillin (800,000 u / 500ml), clarithromycin (1 million u / 500ml) and clindamycin (1 million u / 500ml), shake and wash for 3 Once, 5 minutes each time, wash off the blood and mucus, take...
Embodiment 2
[0061] 1. Raw material preparation:
[0062] 1) Full-term normal delivery of human fetal amniotic membrane,
[0063] 2) 2% ammonia water,
[0064]3) Antibiotics (using penicillin, clarithromycin, clindamycin),
[0065] 4) balanced salt solution (0.9% NACL solution),
[0066] 5) 0.25% trypsin solution,
[0067] 6) 8% concentration of PLLA solution,
[0068] 13) DMEM culture fluid containing 10% serum,
[0069] 14) Co60 radioactive source;
[0070] 2. Select the placenta from 30 minutes to 60 minutes after delivery of the first child of healthy puerpera without infectious diseases and systemic diseases;
[0071] 3. Bluntly separate the amniotic membrane from the placenta under sterile conditions;
[0072] 4. Place the amniotic membrane in balanced salt solution for injection containing penicillin (800,000 u / 500ml), clarithromycin (1 million u / 500ml) and clindamycin (1 million u / 500ml), shake and wash for 3 Once, 5 minutes each time, wash off the blood stains and mucus, t...
Embodiment 3
[0085] 1. Raw material preparation:
[0086] 1) Full-term normal delivery of human fetal amniotic membrane,
[0087] 2) 5% concentration of ammonia water,
[0088] 3) Antibiotics (using penicillin, clarithromycin, clindamycin),
[0089] 4) balanced salt solution (0.9% NACL solution),
[0090] 5) 0.3% trypsin solution,
[0091] 6) 10% concentration of PLLA solution,
[0092] 15) DMEM culture fluid containing 15% serum,
[0093] 16) Co60 radioactive source;
[0094] 2. Select the placenta from 60-90 minutes after delivery of the first child of healthy puerpera without infectious diseases and systemic diseases;
[0095] 3. Bluntly separate the amniotic membrane from the placenta under sterile conditions;
[0096] 4. Place the amniotic membrane in balanced salt solution for injection containing penicillin (800,000 u / 500ml), clarithromycin (1 million u / 500ml) and clindamycin (1 million u / 500ml), shake and wash for 3 Once, 5 minutes each time, wash off the blood stains and m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com