Preparation method of doxofylline
A technology of doxofylline and theophylline, which is applied in the field of drug synthesis, can solve the problems of high risk of registration declaration, lack of control points, unsatisfactory and other problems, and achieve the effects of no high-risk reactions, improved reaction conversion rate, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
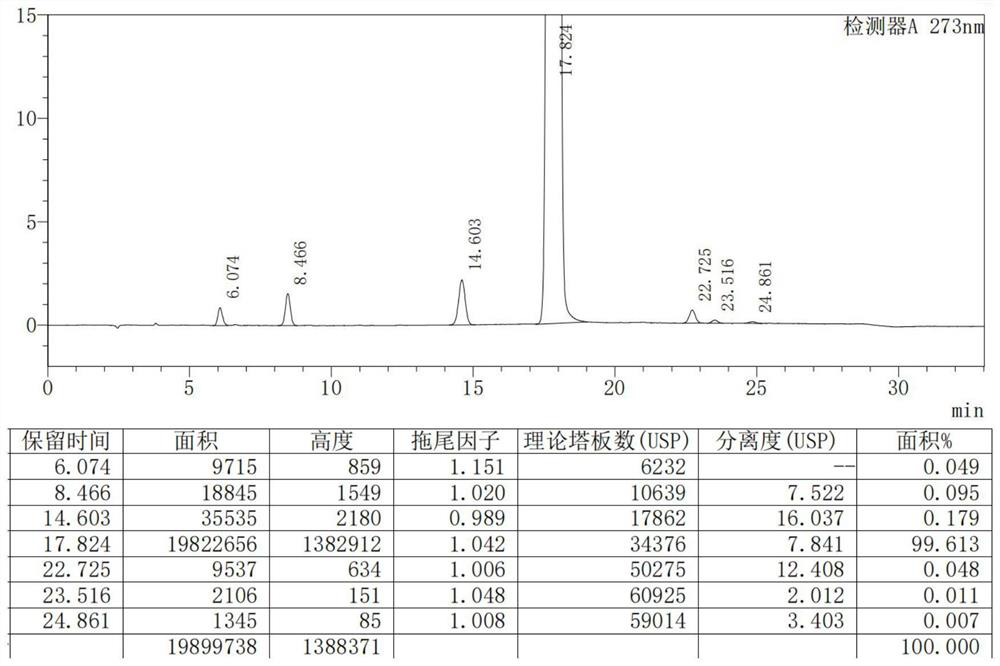
Image
Examples
Embodiment 1
[0041] Preparation of doxofylline:
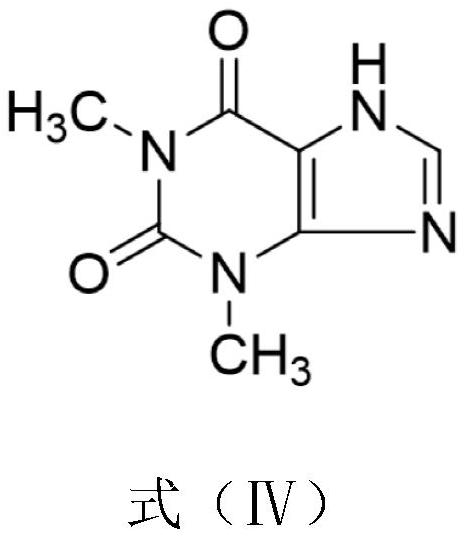
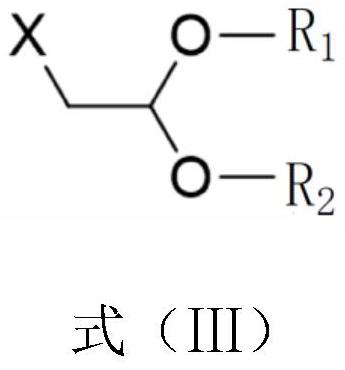
[0042] S1. Add 1000mL of N,N-dimethylformamide into a three-necked flask, add 100g of theophylline, 104.1g of bromoacetaldehyde dimethyl acetal and 84.4g of potassium carbonate successively under stirring, and stir at 120°C for 12h. After completion, filter, concentrate the filtrate under reduced pressure, then add 500mL of methanol, heat up to dissolve, cool down to stir and crystallize, filter, and blow dry at 50°C to obtain an intermediate. The yield of the intermediate is 92.0%;
[0043] S2. Add 400mL of toluene to the three-necked flask, add 100g of intermediates, 34.7g of ethylene glycol, and 3.6g of p-toluenesulfonic acid in turn under stirring, reflux at 110°C for 4 hours, then add 8g of sodium carbonate and mix evenly, filter, and The filtrate was concentrated under reduced pressure, then 400ml of absolute ethanol was added, the temperature was lowered to 25° C., stirred and crystallized for 1 h, filtered, and air-dried at 60° C. t...
Embodiment 2
[0045] Preparation of doxofylline:
[0046] S1. Add 1000mL of N,N-dimethylformamide into a three-necked flask, add 200g of theophylline, 220.5g of bromoacetaldehyde diethyl acetal and 168.8g of potassium carbonate successively under stirring, and stir and react at 125°C for 12h. After completion, filter, concentrate the filtrate under reduced pressure, then add 500mL of methanol, heat up to dissolve, cool down to stir and crystallize, filter, and blow dry at 50°C to obtain an intermediate. The yield of the intermediate is 92.0%;
[0047] S2. Add 400mL of toluene to the three-necked flask, add 100g of intermediates, 34.7g of ethylene glycol, and 3.6g of p-toluenesulfonic acid in turn under stirring, reflux at 110°C for 4 hours, then add 8g of sodium carbonate and mix evenly, filter, and The filtrate was concentrated under reduced pressure, then 400ml of absolute ethanol was added, the temperature was lowered to 25° C., stirred and crystallized for 1 h, filtered, and air-dried a...
Embodiment 3
[0049] Preparation of doxofylline:
[0050] S1. Add 1000mL N,N-dimethylformamide into the three-necked flask, add 95g of theophylline, 95g of bromoacetaldehyde dimethyl acetal and 76g of potassium carbonate successively under stirring, and stir and react at 115°C for 14h. After the reaction is completed, Filtrate, concentrate the filtrate under reduced pressure, then add 500mL of methanol, heat up to dissolve, cool down to stir and crystallize, filter, and blow dry at 50°C to obtain an intermediate, the yield of which is 92.2%;
[0051] S2. Add 400mL of toluene to the three-necked flask, add 95g of intermediates, 28.5g of ethylene glycol, and 2.85g of p-toluenesulfonic acid in turn under stirring, and reflux at 105°C for 5 hours, then add 4g of sodium carbonate and mix evenly, filter, and The filtrate was concentrated under reduced pressure, then 400ml of absolute ethanol was added, the temperature was lowered to 20° C., stirred and crystallized for 1 h, filtered, and air-drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com