Preparation method for chiral piperylhydrazine compound and recycling method for chiral resolving agent
A technology of chiral resolution and piperidine amine, which is applied in the fields of organic chemistry and organic chemistry, and can solve the problems that are not conducive to large-scale industrial production, the use of chiral resolving agents is large, and the generation of industrial solid waste is large. , to achieve the effect of easy control of purity, easy operation and control of impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
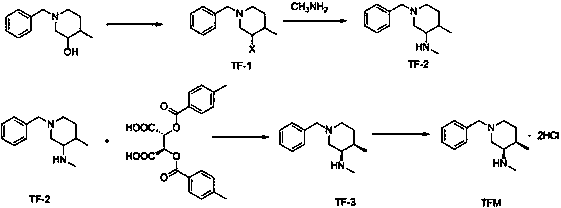
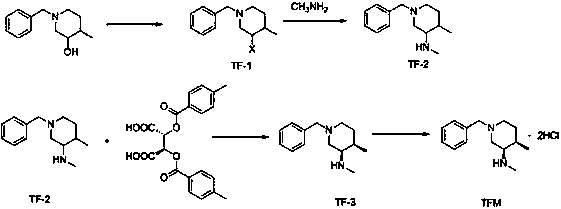
[0021] Preparation of intermediate TF-1: Add 1-benzyl-4-methyl-3-piperidinol (30.8g, 0.15mol) and 450mL dichloromethane to the reaction flask, stir to dissolve, cool to 0-5 ℃, then add thionyl chloride (71.4g, 0.6mol, 4.0eq), stir for 1h, then raise the temperature to 25-30℃ for reaction, and monitor the reaction by TLC. After the reaction is complete, add 500mL of water, then add potassium carbonate (41.4g, 0.3mol, 2.0eq), adjust the pH value to alkaline, let stand to separate the liquid, extract the aqueous layer with dichloromethane (200mL×2), and combine the organic phases , washed with saturated sodium chloride (500mL×1), dried over anhydrous sodium sulfate, filtered with suction, and evaporated to dryness under reduced pressure to obtain 31.2g of an oily product with a yield of 93%.
[0022] Preparation of intermediate TF-2: Add intermediate TF-1 (31.2g, 0.14mol) and 300mL ethanol to the reaction flask, stir to dissolve, then add potassium carbonate (19.3g, 0.14mol, 1.0q...
Embodiment 2
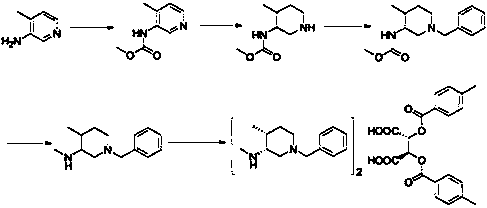
[0027] Preparation of intermediate TF-1: Add 1-benzyl-4-methyl-3-piperidinol (41g, 0.2mol) and 600mL dichloromethane into a reaction flask, stir to dissolve, and cool down to 0-5°C , and then add phosphorus oxychloride (61.32g, 0.4mol, 2.0eq), stir the reaction for 1h, then raise the temperature to 25-30°C for reaction, and monitor the reaction by TLC. After the reaction is complete, add 600mL of water, then add potassium carbonate (55.2g, 0.4mol, 2.0eq), adjust the pH value to alkaline, let stand to separate the liquid, extract the aqueous layer with dichloromethane (300mL×2), and combine the organic phases , washed with saturated sodium chloride (500mL×1), dried over anhydrous sodium sulfate, filtered with suction, and evaporated to dryness under reduced pressure to obtain 42.3g of an oily product with a yield of 95%.
[0028] Preparation of intermediate TF-2: Add intermediate TF-1 (42.3g, 0.19mol) and 400mL methanol to the reaction flask, stir to dissolve, then add sodium c...
Embodiment 3
[0033] Preparation of intermediate TF-1: Add 1-benzyl-4-methyl-3-piperidinol (21g, 0.1mol) and 200mL dichloromethane into a reaction flask, stir to dissolve, and cool down to 0-5°C , and then add hydrobromic acid (24.7g, 0.3mol, 3.0eq), stir the reaction for 1h, then raise the temperature to 25-30°C for reaction, and monitor the reaction by TLC. After the reaction is complete, add 300mL of water, then add potassium carbonate (28.6g, 0.2mol, 2.0eq), adjust the pH value to alkaline, let stand to separate the layers, extract the aqueous layer with dichloromethane (200mL×2), and combine the organic phases , washed with saturated sodium chloride (300mL×1), dried over anhydrous sodium sulfate, filtered with suction, and evaporated to dryness under reduced pressure to obtain 20.9g of an oily product with a yield of 92%.
[0034] Preparation of intermediate TF-2: Add intermediate TF-1 (20.9g, 0.09mol) and 300mL ethanol to the reaction flask, stir to dissolve, then add potassium carbon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com