Method for preparing ziconotide
A technology of ziconotide and solid-phase synthesis, which is applied in the field of peptide synthesis, can solve the problems of unfavorable high purity, increased impurities, unfavorable and other problems, achieve wide application prospects, improve reaction efficiency, and avoid the effect of impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
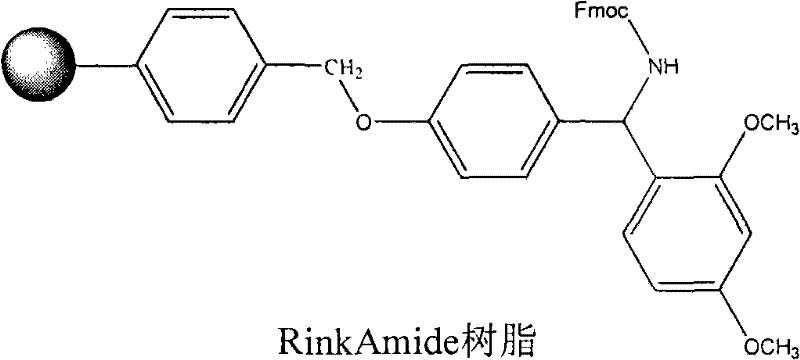
[0038] Embodiment 1: the synthesis of Fmoc-Cys(Acm)-Rink Amide resin
[0039] Rink Amide resin 14.3g, substitution degree is 0.7mmol / g, joins in the solid-phase reaction column, after adding DCM to swell the resin for 30 minutes, remove Fmoc with 20% hexahydropyridine / DMF, wash 6 times with DMF, the 1.87 gFmoc-Cys(Acm)-OH, 1.71gHATU, 0.73gHOAt were dissolved in DMF under ice-cooling conditions, added to the above resin and reacted for 10min, then added 1.2ml TMP, and reacted at room temperature for 45min. After washing 3 times with DMF, 3 times with DCM, and 3 times with methanol for 3 min, 5 min and 8 min respectively, the resin was shrunk to obtain Fmoc-Cys(Acm)-RinkAmide resin, and the detected substitution degree was 0.4 mmol / g. The route is as follows:
[0040]
Embodiment 2
[0041] Embodiment 2: the synthesis of Fmoc-Cys(Acm)-Rink Amide MBHA resin
[0042] Add 13.8 g of Rink Amide MBHA resin, with a substitution degree of 0.6 mmol / g, into the solid-phase reaction column, add DCM to swell the resin for 30 minutes, remove Fmoc with 20% hexahydropyridine / DMF, wash with DMF 6 times, and 1.87g Fmoc-Cys(Acm)-OH, 1.71g HATU, 0.73g HOAt were dissolved in DMF under ice-cooling conditions, added to the above resin and reacted for 10min, then 1.2ml TMP was added and reacted at room temperature for 45min. After washing 3 times with DMF, 3 times with DCM, and 3 times with methanol for 3 min, 5 min and 8 min respectively, the resin was shrunk to obtain Fmoc-Cys(Acm)-Rink Amide MBHA resin, and the detected substitution degree was 0.35 mmol / g.
Embodiment 3
[0043] Embodiment 3: the synthesis of Fmoc-Cys(Acm)-Rink Amide BHA resin
[0044] With Rink Amide BHA resin 15.6g, substitution degree is 0.6mmol / g, joins in the solid-phase reaction column, after adding DCM swelling resin 30 minutes, remove Fmoc with 20% hexahydropyridine / DMF, wash 6 times with DMF, will 1.87g Fmoc-Cys(Acm)-OH, 1.71g HATU, 0.73g HOAt were dissolved in DMF under ice-cooling conditions, added to the above resin and reacted for 10min, then 1.2ml TMP was added and reacted at room temperature for 45min. After washing with DMF for 3 times, washing with DCM for 3 times, and using methanol for 3 minutes, 5 minutes and 8 minutes respectively, the resin was shrunk to obtain Fmoc-Cys(Acm)-Rink Amide BHA resin, and the detected substitution degree was 0.37mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com