ROR gamma t activity inhibition compound and preparation method and application thereof
A technology of activity inhibition and compounds, applied in the field of RORγt activity inhibition compounds and their preparation, can solve the problems of not finding target drugs, reducing the differentiation ability of Th17 cells, and weakening clinical symptoms, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
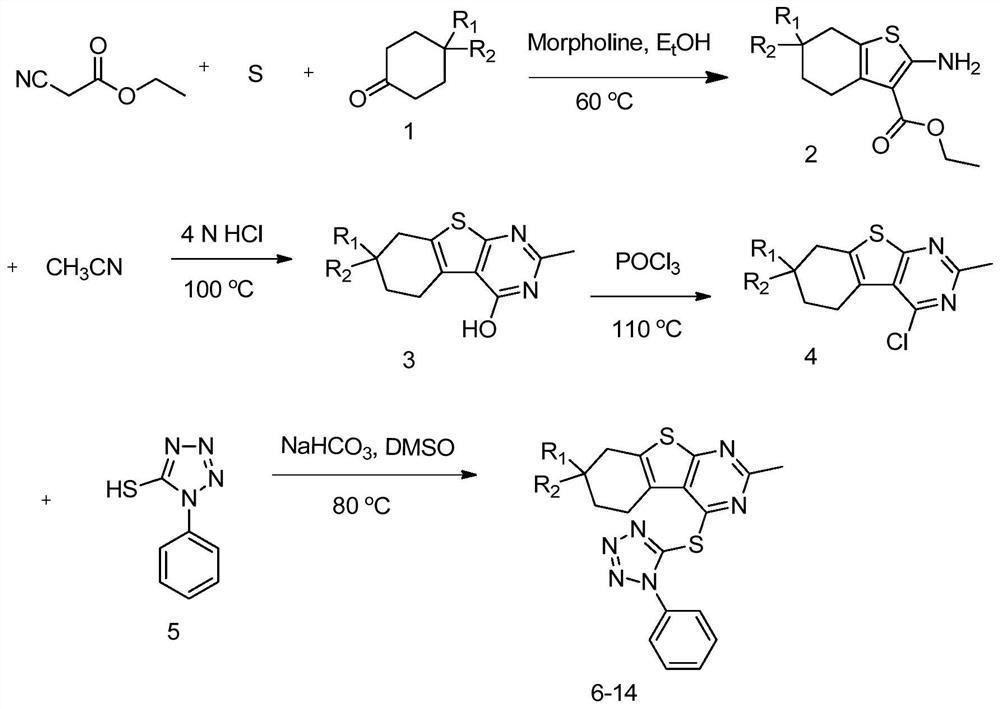
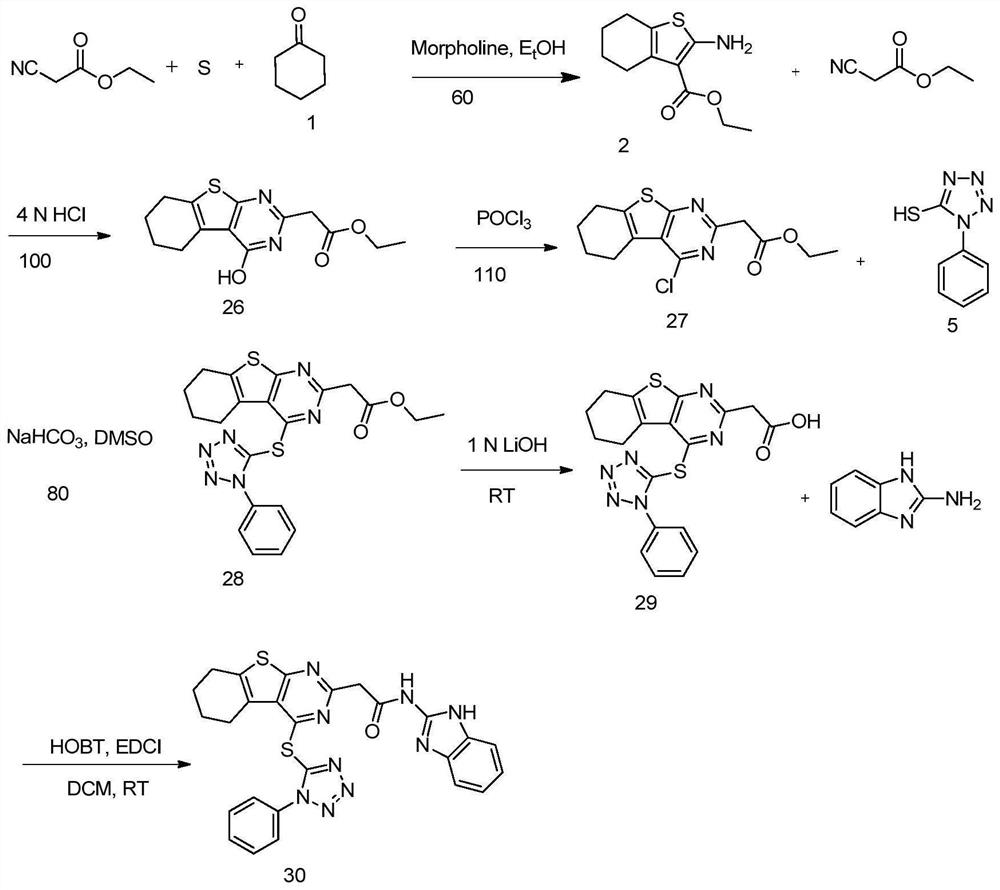
[0051] The present invention also provides a method of preparing a RORγT activity inhibiting compound comprising the steps of:
[0052] Compound compound And the sulfur monomer reacts under the conditions of matrine and ethanol, preparing a compound
[0053] Preparative compound NC-R 3 Preparation of compounds in 1,4-dioxidin solution in hydrogen chloride
[0054] Preparative compound Preparation of compounds with trichloroside
[0055] Preparative compound Compound Reaction preparation compound under the presence of sodium bicarbonate and dimethyl sulfoxide
[0056] In a specific example, compounds It is prepared by reacting thiocyanate phenyl and laminated sodium in aqueous solution.
[0057] More specifically, R 1 With r 2 One of them is H, the other is
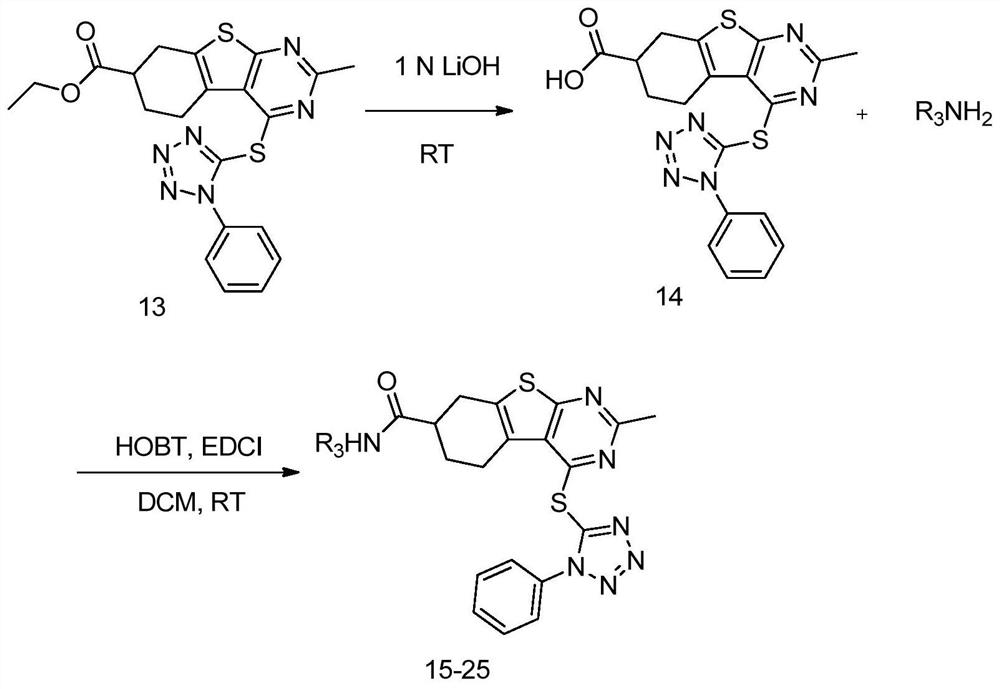
[0058] The preparation method also includes compounds Reaction preparation compound in a lithium hydroxide solution A step of.
[0059] In particular, R 1 With r 2 One of them is H, the other is
[0060] The prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com