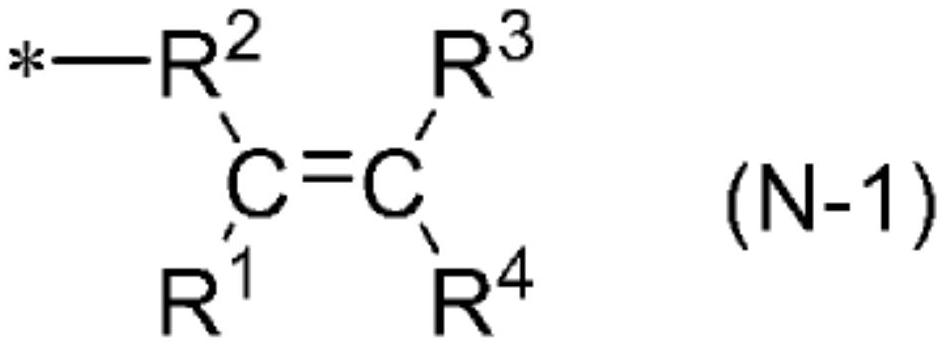
Liquid crystal light control element
A liquid crystal dimming and component technology, which is applied in optics, nonlinear optics, instruments, etc., can solve the problem of voltage retention characteristics being damaged, and achieve the effects of voltage retention reliability and excellent sealing tightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0207] The following examples are given to illustrate the present invention in more detail, but the explanation of the present invention is not limited to these examples. In addition, the meaning of the abbreviation etc. of the compound used below is as follows.
[0208] (tetracarboxylic dianhydride)
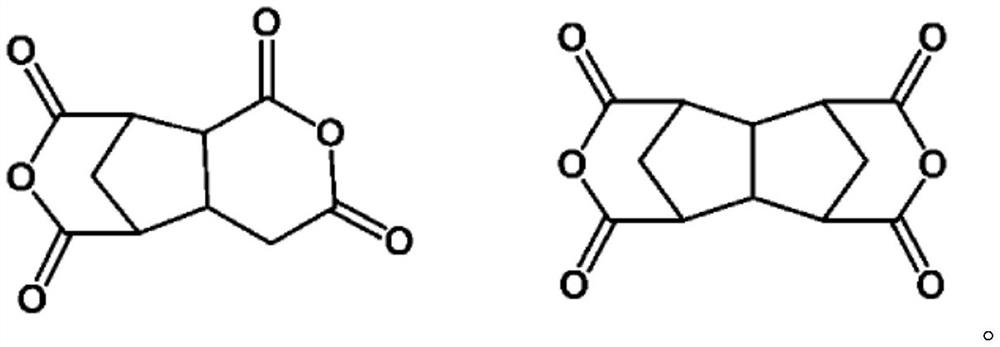
[0209] CA-1: 1,2,3,4-cyclobutanetetracarboxylic dianhydride
[0210] CA-2: Bicyclo[3.3.0]octane-2,4,6,8-tetracarboxylic dianhydride
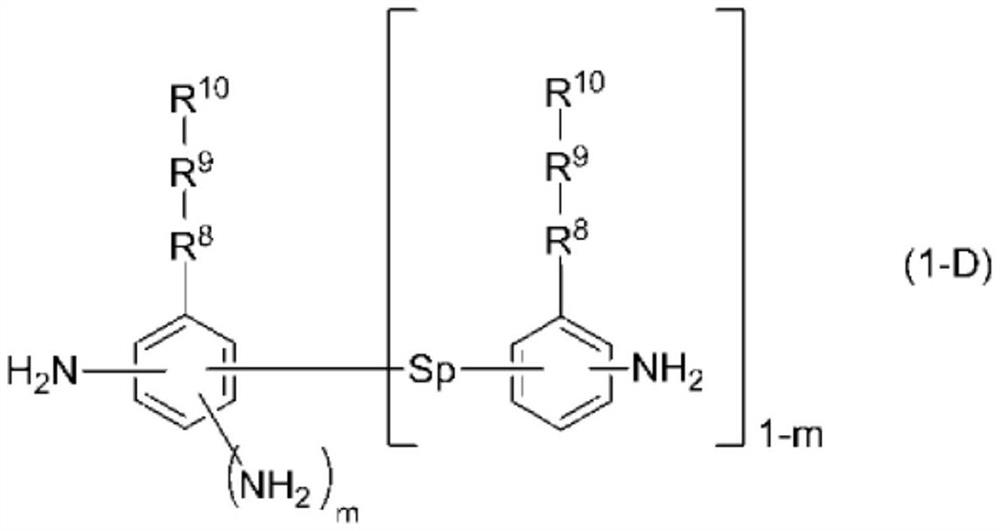
[0211] (diamine compound)
[0212] DA-1: 3-aminobenzylamine
[0213] DA-2: N,N-Diallyl-2,4-diaminoaniline
[0214] DA-3: Diamine represented by the following formula DA-3
[0215] DA-4: 1,3-Diamino-4-{4-[trans-4-(trans-4-n-pentylcyclohexyl)cyclohexyl]phenoxy}benzene
[0216]
[0217] The structure of (B) component used in the Example and the comparative example is as follows.
[0218]
[0219] (Organic solvents)
[0220] GBL: γ-butyrolactone,
[0221] PGME: Propylene Glycol Monomethyl Ether
[0222] (Molecular Weight Determination)...
Synthetic example 1
[0254] Make the atmosphere in a 50mL (liter) four-necked flask with a stirring device nitrogen, measure DA-1 1.12g (9.20mmol) DA-2 1.87 (9.20mmol) and DA-4 2.00g (4.60mmol), add 39.5 g of NMP was stirred and dissolved while feeding nitrogen gas. While stirring the diamine solution, 2.88 g (11.5 mmol) of CA-2 was added, and after reacting at 60° C. for 3 hours, CA-1 (2.25 g, 10.8 mmol) and NMP (4.39 g) were added, and at 40 The reaction was carried out at °C for 6 hours to obtain a polyamic acid solution (PAA-1) having a resin solid content concentration of 20.0% by mass. The viscosity of this polyamic acid solution was 660.1 mPa·s. Mn of this polyamic acid was 12,426, and Mw was 41,548.
[0255]
[0256] Polyamic acids (PAA-2) to (PAA-5) were obtained by performing the same operation as in Synthesis Example 1 using the components shown in the following Table 1, respectively. The viscosity and molecular weight of the obtained polyamic-acid solution are shown in Table 1 bel...
Synthetic example 6
[0258] After adding NMP to the polyamic acid solution (2) (50.0 g) obtained in Synthesis Example 1 and diluting to 7.50% by mass, acetic anhydride (6.26 g) and pyridine (19.4 g) were added as an imidation catalyst, °C for 3 hours. This reaction solution was poured into methanol (556 ml), and the resulting precipitate was filtered off. This deposit was washed with methanol, and it dried under reduced pressure at 100 degreeC, and obtained the polyimide powder (PI-1). The imidization rate of this polyimide was 51%. Mn of this polyimide powder was 11,201, and Mw was 32,594.
[0259]
[0260] Polyimide powders (PI-2) to (PI-5) were obtained by performing the same operation as in Synthesis Example 6 using the components shown in the following Table 2, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Film thickness | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com