Kit, reaction system and method for detecting pneumocystis jirovecii and drug resistance of pneumocystis jirovecii
A reaction system and technology for spore bacteria, which are applied in the directions of microorganism-based methods, biochemical equipment and methods, and the determination/inspection of microorganisms, which can solve the problems of lack of established drug resistance detection methods and low attention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
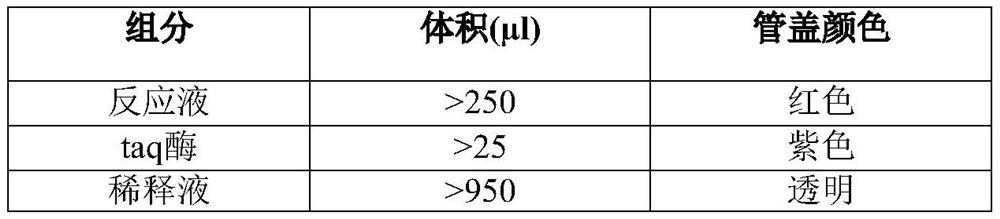
[0050] This embodiment is an example of a kit for detection of Pneumocystis sp. and drug resistance. The kit components of this embodiment are shown in Table 1, and the specification is 25 tests / box.
[0051] Table 1 Pneumocystis and drug resistance detection kit composition
[0052]
[0053]
[0054] The test kit of this embodiment is suitable for the following instruments:
[0055] 480II (Roche)
[0056] Q(QIAGEN)
[0057] CFX96 (Biorad)
[0058] Mic qPCR (Bio Molecular Systems)
[0059] Quantstudio 5 (Thermo Fisher Scientific)
[0060] The storage conditions of the Pneumocystis and Drug Resistance Detection Kit are -15°C to -30°C and protected from light; try to avoid repeated freezing and thawing (no more than 10 times); see the product label for the expiration date. To avoid cross-contamination, it is recommended to operate in a PCR laboratory and store positive controls separately.
[0061] The Mastermix in the kit contains the composition shown in Table...
Embodiment 2
[0065] 1. Nucleic acid extraction
[0066] If the sample is viscous, pretreat the sample with DTT. Add 10% 1M DTT to the samples and incubate at 37°C for 15 minutes. use Blood minikit (QIAGEN) or Extract sample nucleic acid, see instructions for details. Note: Add IC to the sample before nucleic acid extraction.
[0067] Avoid repeated freezing and thawing of the extracted nucleic acid. If it is used on the day of extraction, it is recommended to store the DNA at 4°C, and it can also be stored at -20°C for a long time.
[0068] 2. Operation steps
[0069] 2.1 Preparation of reaction system
[0070] Prepare the PCR reaction system according to Table 3. After the preparation is complete, mix well, dispense 20 μl into PCR reaction wells, and place the reaction plate on ice.
[0071] Table 3 Preparation of Pneumocystis and Drug Resistance Detection Kit Reaction Mixture
[0072] component name Volume (1×) Volume (10×) The reaction solution 10 100 ...
Embodiment 3
[0102] This embodiment is a performance test example of the kit.
[0103] 1. Minimum detection limit
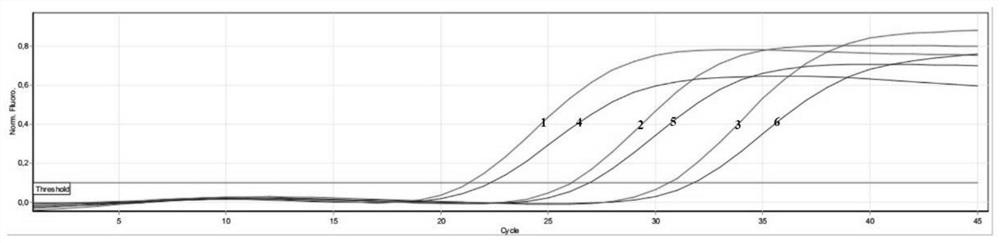
[0104] PCR amplification was performed using a DNA fragment containing mtLSU and DHPS sequences as a template. The template was diluted in a 10-fold gradient with concentrations of 1000copies / μL, 100copies / μL, 10copies / μL, 1copies / μL, and 0.1copies / μL, and the minimum detection limit was determined by the detection rate of ≥95% in 20 repeated tests.
[0105] category LOD(copies / μL) DHPS 1 wxya 1
[0106] 2. Specificity
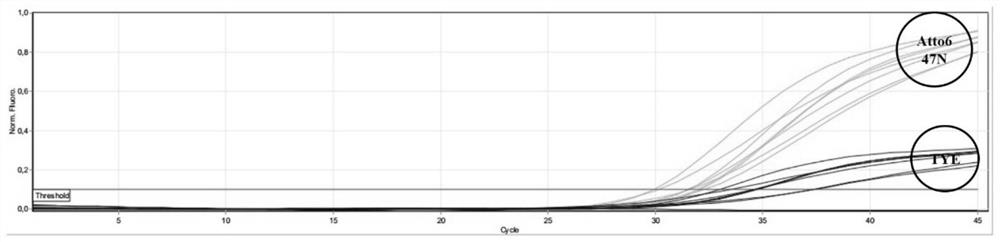
[0107] In this example, 21 fungal samples of different types were used, and the above-mentioned fungal samples were detected using the kit in Example 1. Only the Pneumocystis sp. samples were tested positive, and the specificity reached 100%.
[0108] fungal type wxya DHPS Pneumocystis + + Cryptococcus neoformans - - Saccharomyces cerevisiae - - Bai Nian - - Candida parapsilosis - ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com