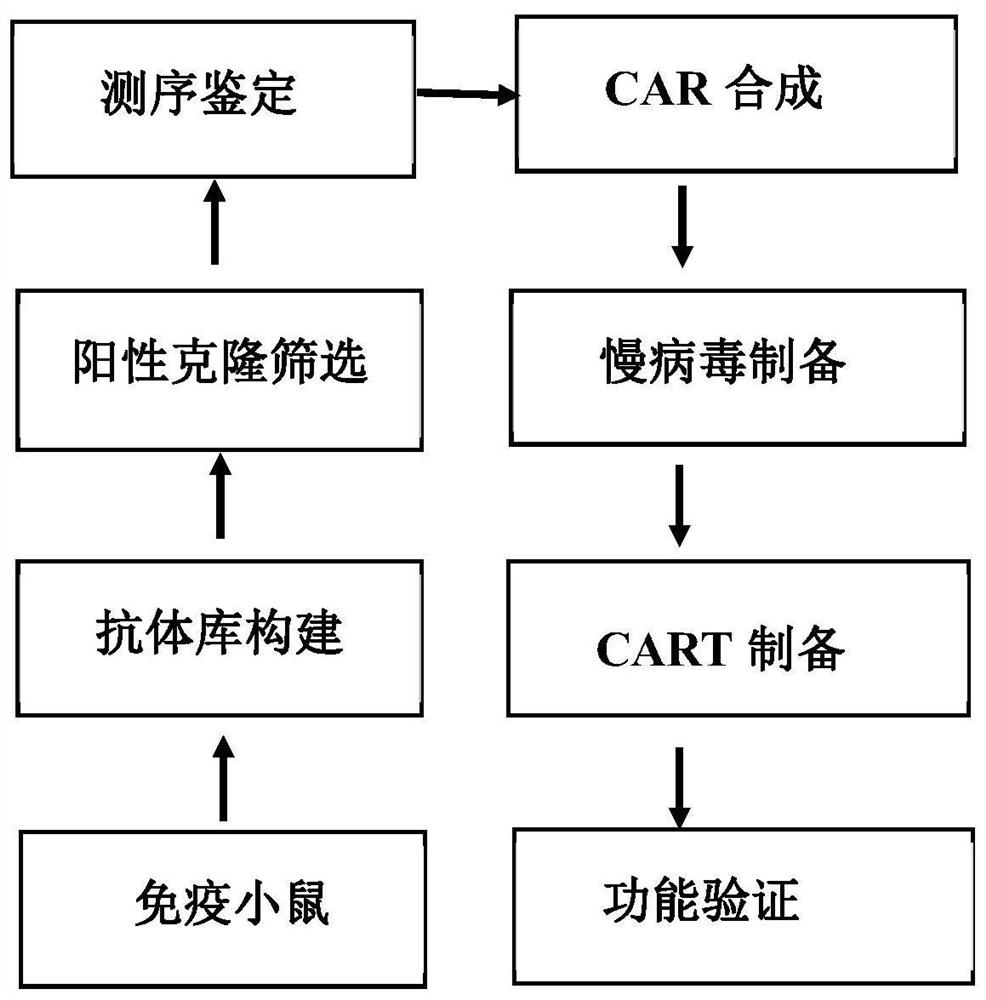
A kind of cd123 binding protein, car containing it and application thereof
A technology for binding proteins and variable regions, which is applied in the field of CAR and CD123 binding proteins, and can solve the problems of normal hematopoietic cytotoxicity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1: Production and immunoassay of murine monoclonal antibodies
[0148] 1.1 Immune mice and serum affinity ELISA detection
[0149] In order to generate antibodies against CD123, in this example, CD123 protein (No. SNS001-002, purchased from ACRO, Beijing Baipsis Biotechnology Co., Ltd.) was used to immunize mice.
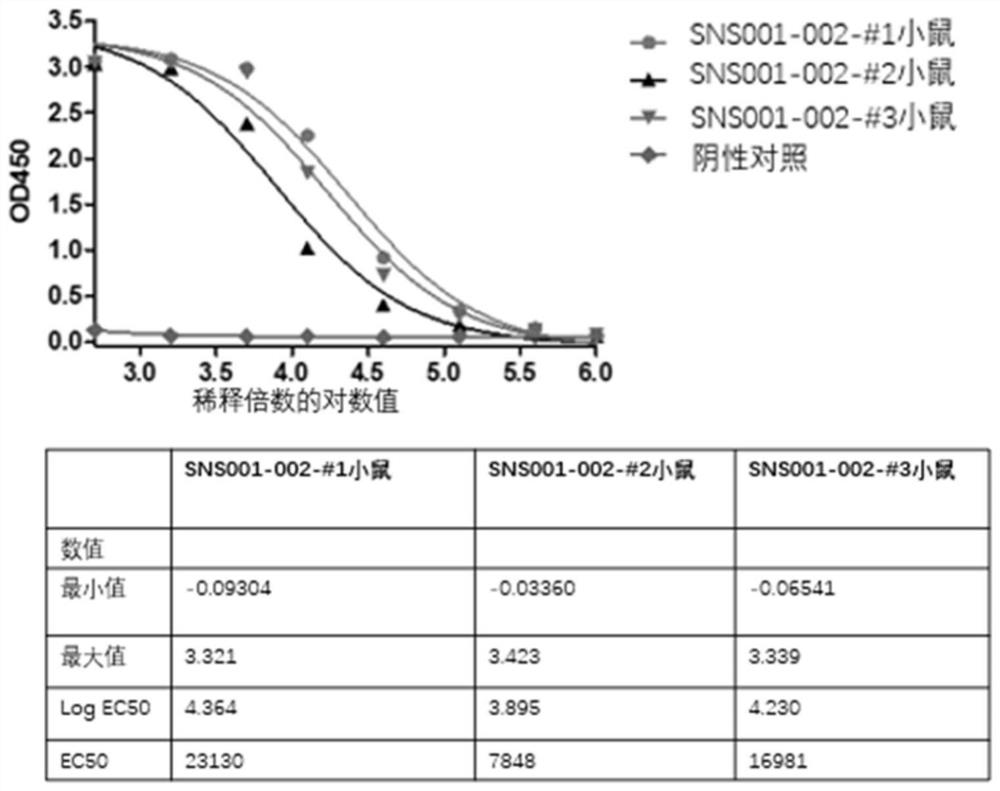
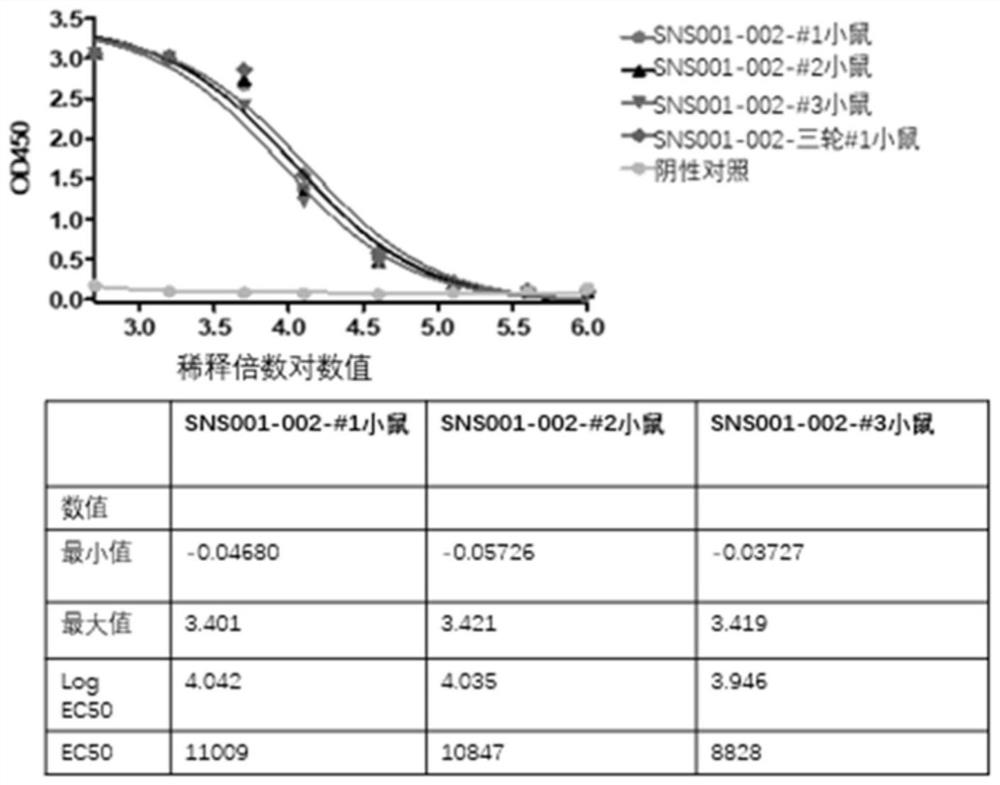
[0150] Three mice were immunized by subcutaneous injection, and after three rounds of immunization, serum samples were obtained by blood sampling through the tail vein. Affinity ELISA was performed on the serum of the third immunized mice.
[0151] Specific steps are as follows:
[0152] 1) Antigen coating: Use affinity Elisa plate, add SNS001-002 diluted with PBS to 2 μg / ml in 96-well semi-well microtiter plate, 30 μl per well, and coat overnight at 4 degrees.
[0153] 2) Wash the plate: wash the plate 3 times with PBST.
[0154] 3) Blocking: 5% PBSM, room temperature, 2 hours.
[0155] 4) Wash the plate: wash the plate 3 times with TBST.
[01...
Embodiment 2
[0271] Example 2: Construction and virus packaging of lentiviral plasmids containing chimeric antigen receptors
[0272] 2.1 Synthesis of Chimeric Antigen Receptor Nucleic Acid Sequence
[0273] In this embodiment, the arrangement order of the chimeric antigen receptor sequence is designed as follows: from the 5th end to the 3rd end: leading chain-scFv-CD8 hinge region-CD8 transmembrane region-4-1BB-CD3ζ.
[0274] Use the light chain variable region and heavy chain variable region sequences (VL, VH sequence numbers in Table 11) of the 18 kinds of positive clones screened in Example 1 and submit the following sequences at the same time, respectively numbered as 32716 clones (from the United States Hope City) light and heavy chain variable region sequence (from patent CN201480024929.6), leading chain region (SEQ ID NO:73), linker region (SEQ ID NO:75), CD8 hinge region (SEQ ID NO:77), CD8 transmembrane region (SEQ ID NO:79), 4-1BB co-stimulatory region (SEQ ID NO:81) and CD3ζ s...
Embodiment 3
[0297] Example 3: Construction and verification of stable expression cell lines of K562-CD123
[0298] First, NCBI (https: / / www.ncbi.nlm.nih.gov / ) searched for the CD123 protein sequence (XP_016884980.1), commissioned Nanjing GenScript to synthesize a lentiviral plasmid vector containing the protein gene, and transfected into competent For cell stb13, the plasmid was extracted according to Example 2.2. The extracted target plasmid and packaging plasmid were transfected into 293T cells according to a certain ratio, and the CD123 virus liquid was collected and filtered after 48 hours and 72 hours of transfection respectively, and referred to Example 2.4 Perform virus titration.
[0299] According to MOI=5:1, K562 cells (from Peking University Medical Center) were infected with CD123 virus solution. Puromycin selection was carried out 2 days after infection (the screening concentration was 5 μg / ml). One week after the selection, unstained K562 cells were used as control cells, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com