Coronary drug-coated balloon
A drug coating and balloon technology, applied in coatings, balloon catheters, catheters, etc., can solve the problems of increasing patient pain, increasing contact area, secondary injury to patients, etc. The effect of avoiding secondary damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] In this application, the preparation method of TPU refers to the following steps:
[0042] (1) Add polylactic acid to N,N-dimethylformamide, after the polylactic acid dissolves completely and becomes transparent, then add isophorone diisocyanate, heat up to 50-70°C under nitrogen atmosphere, and react 30-90 minutes;
[0043](2) in step (1), add 1,4-butanediol, fully mix, detect reaction end point with infrared spectrum, treat to be unable to detect NCO (the absorption peak of NCO group is 2226cm -1 ), the reaction ends.
[0044] In some preferred embodiments, the polylactic acid has a weight average molecular weight of 60,000.
[0045] Polylactic acid, product number LM1221, was purchased from Shanghai Lianmai Bioengineering Co., Ltd.
[0046] In some preferred embodiments, the weight ratio of POM to TPU is 1:0.5-2.
[0047] In this application, the combination of POM and TPU can be used to solve the problems of low notched impact strength and poor toughness of POM ...
Embodiment 1
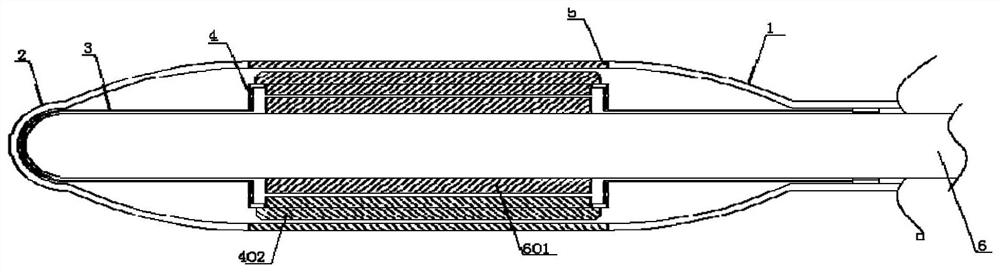
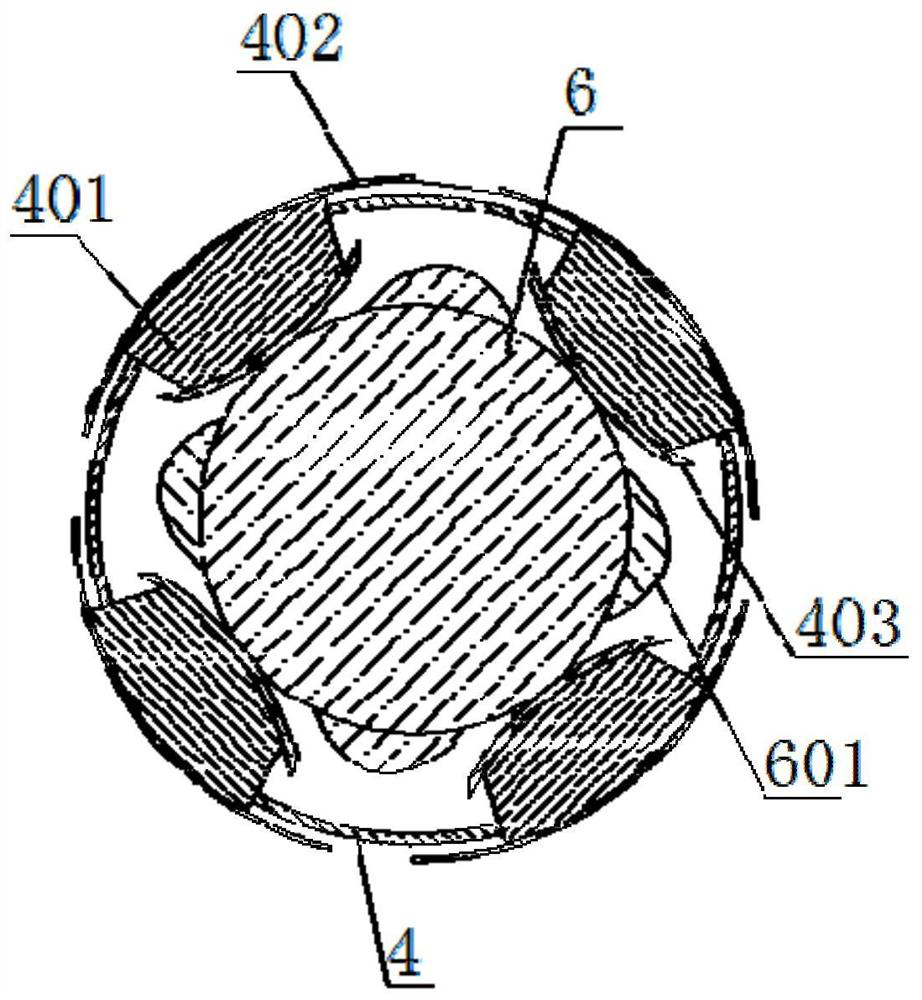
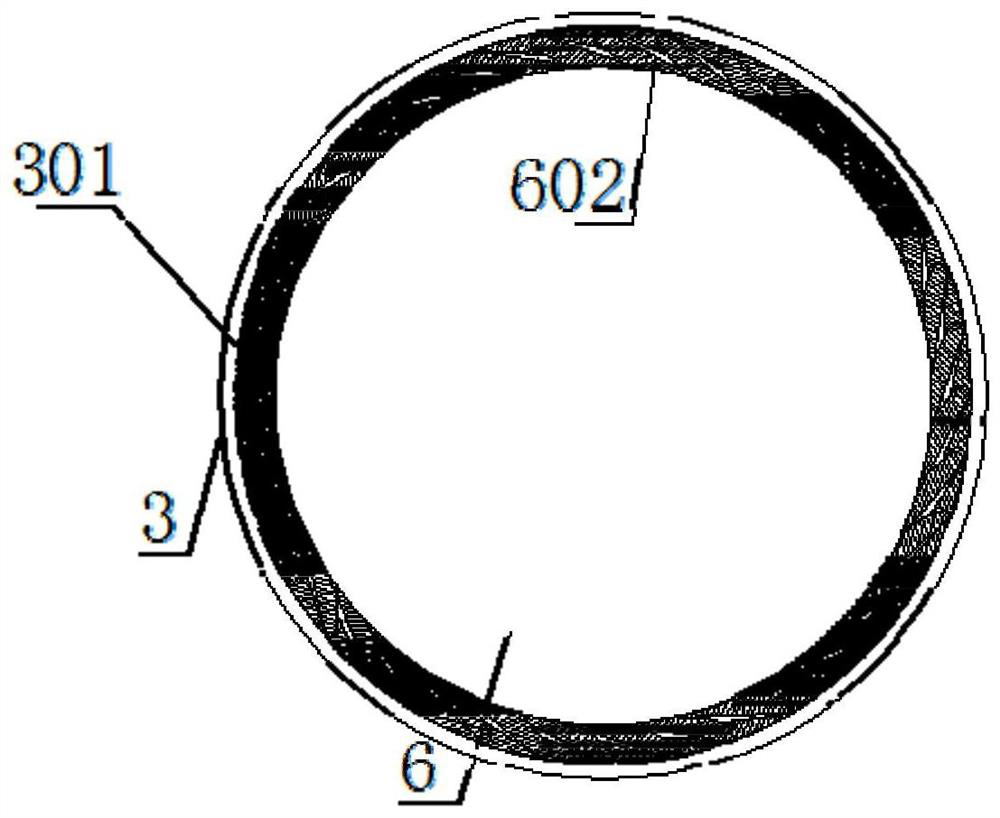
[0080] see Figure 1-3 , the present invention provides a coronary drug-coated balloon, comprising a balloon body 1, an introduction head 2 is arranged on the left side of the balloon body 1, and a reference column 3 is arranged in the inner cavity of the introduction head 2, and the reference column 3 The middle part is provided with a compensation chamber 4, the middle part of the outside of the balloon body 1 is provided with a coating 5, the inner cavity of the reference column 3 is connected with a fine-tuning shaft 6, and the outer ring of the compensation chamber 4 is equidistantly connected with a support piece 401, and the support piece 401 is fixedly welded with a support arc 402 at one end located outside the compensation chamber 4, and is fixedly welded with a trimming tile 403 at one end of the support plate 401 located in the inner cavity of the compensation chamber 4, and the fine-tuning shaft 6 is fixedly welded at an equal distance up the outer ring of the inne...
Embodiment 2
[0082] A coronary drug-coated balloon, the preparation raw materials are calculated in parts by weight, including: 50 parts of POM, 50 parts of TPU, 0.1 part of modified nano-zinc oxide, 0.2 part of chitosan, 0.1 part of peppermint extract, and geranium extract 0.1 part.
[0083] The average particle size of the modified nano zinc oxide is 60nm.
[0084] POM, brand N2720 M63, was purchased from BASF, Germany; chitosan, brand C804726, deacetylation degree ≥ 95%, was purchased from Shanghai Macklin Biochemical Technology Co., Ltd.
[0085]The raw materials for TPU preparation include, in parts by weight, 20 parts of polylactic acid, 45 parts of isophorone diisocyanate, 12 parts of 1,4-butanediol, and 150 parts of N,N-dimethylformamide.
[0086] The weight-average molecular weight of polylactic acid is 60000, and the article number is LM1221, purchased from Shanghai Lianmai Bioengineering Co., Ltd.
[0087] The preparation method of TPU refers to the following steps:
[0088] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com