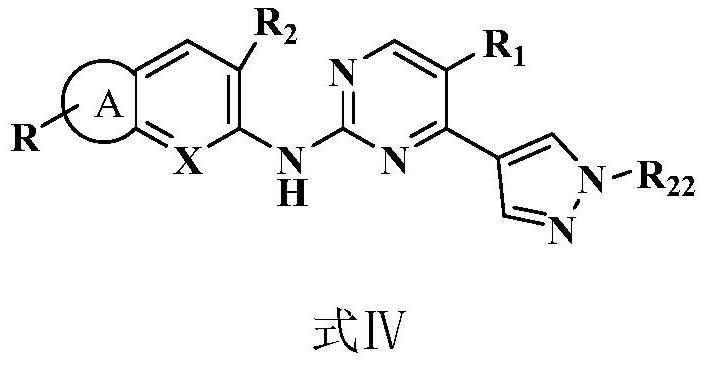
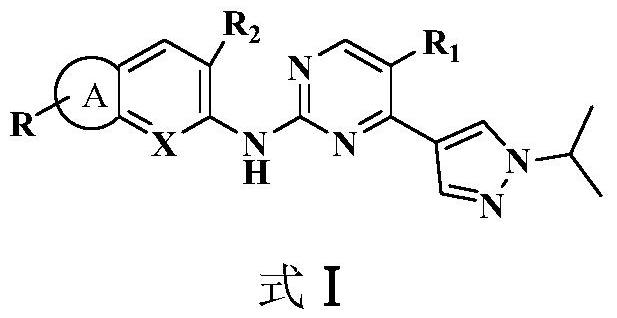
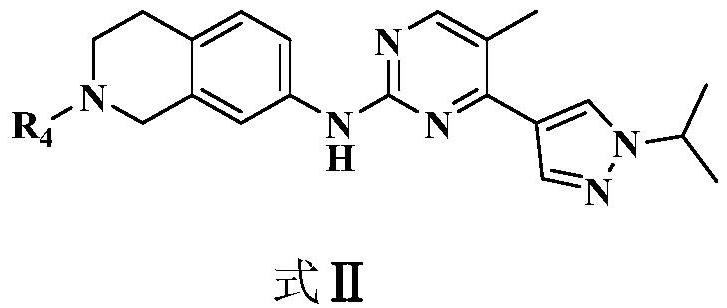
2,4-disubstituted pyrimidine derivative as well as preparation method and application thereof
A pyrimidine derivative and di-substitution technology, applied in the field of chemical medicine, can solve the problems of poor enzyme activity and low oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Example 1 6-hydroxypropyl-N-(4-(1-isopropyl-1H-pyrazol-4-yl)5-methylpyrimidin-2-yl)-5,6,7,8-tetra Preparation of hydronaphthyridine-2-amine (CLJ-100)
[0160]
[0161] Step 1: Synthesis of 1-propylpyrazole-4-boronic acid pinacol ester (compound of formula 2)
[0162] Add 4-boronic acid pinacol ester (1.9g, 10mmol), 2-iodopropane (3.4g, 20mmol), cesium carbonate (6.5g, 20mmol) into acetonitrile (50ml), and react in an oil bath at 80°C 2 hours. After the reaction is completed, filter while hot and concentrate the filtrate to obtain the compound of formula 2.
[0163] Step 2: Synthesis of 2-chloro-5-methyl-4-(1-isopropyl-1H-pyrazol-4-yl)pyrimidine (compound of formula 3) Formula 2 compound (1.9g, 10mmol), 2 , 4-dichloro-5-fluoropyrimidine (1.7g, 10mmol), potassium carbonate (3.4g, 25mmol) and dppf (Pd2Cl2) (0.75g, 1mmol) join in the 250mL three-necked flask, add dioxane / Ethanol / water=7:3:4 (total 70mL) was used as solvent, nitrogen was replaced three times, and the...
Embodiment 2
[0170] Example 2 2-Hydroxyethyl-N-(4-(1-isopropyl-1H-pyrazol-4-yl)5-methylpyrimidin-2-yl)-1,2,3,4-tetra Preparation of Hydroisoquinoline-6-Aminol (CLJ-101)
[0171]
[0172] The synthesis of CLJ-101 is the same as in Example 1, with 6-amino-N-Boc-1,2,3,4-tetrahydroisoquinoline replacing 2-amino-N-Boc-5,6,7,8-tetrahydro Hydronaphthyridine, 2-iodoethanol can replace 3-bromo-1-propanol to obtain the final product CLJ-101. 1 H NMR (400MHz, DMSO-d 6 )δ:9.20(s,1H),8.29(d,J=22.7Hz,2H),8.08(s,1H),7.65(d,J=2.3Hz,1H),7.46(dd,J=8.3,2.3 Hz,1H),6.94(d,J=8.4Hz,1H),4.62(h,J=6.6Hz,1H),4.46(s,1H),3.67–3.46(m,4H),2.80(d,J =5.8Hz, 2H), 2.71(t, J=5.8Hz, 2H), 2.57(t, J=6.2Hz, 2H), 2.31(s, 3H), 1.48(d, J=6.7Hz, 6H). 13 C NMR(101MHz,DMSO)δ:159.88,159.00,157.63,139.42,139.31,134.44,129.38,127.92,126.71,120.74,118.31,116.72,116.35,60.74,59.30,56.07,53.83,51.64,29.63,23.03,17.15 .m / z:393.5070[M+H] + .
Embodiment 3
[0173] Example 3 2-Hydroxyethyl-N-(4-(1-isopropyl-1H-pyrazol-4-yl)5-methylpyrimidin-2-yl)-1,2,3,4-tetra Preparation of Hydroisoquinolin-7-fluoro-6-amine (CLJ-102)
[0174]
[0175] The pyrimidine part of CLJ-102 was synthesized as in Example 2;
[0176] Aromatic amines are partially synthesized as follows:
[0177] Step 1: Synthesis of 4-fluorophenethylamine trifluoroacetamide (compound of formula 2)
[0178] Dissolve 4-fluorophenethylamine (7g, 50mmol) and triethylamine (13g, 125mmol) in dichloromethane (100mL), add trifluoroacetic anhydride (12.6g, 60mmol) dropwise, react at room temperature for 30min, add a large amount of Water (about 200 mL) was stirred vigorously, separated, the organic phase was concentrated by distillation under reduced pressure, and slurried with ether to obtain the compound of formula 2.
[0179] Step 2: Synthesis of 7-fluoro-1,2,3,4-tetrahydroisoquinoline trifluoroacetamide (compound of formula 3)
[0180] Dissolve 4-fluorophenethylamine trif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com