A kind of isolator and hydrogen peroxide gas sterilization method
A hydrogen peroxide and isolator technology, applied in chemistry, disinfection, etc., can solve the problems of weak reliability in the sterilization process, and achieve the effects of enhanced reliability, good reliability, and enhanced reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
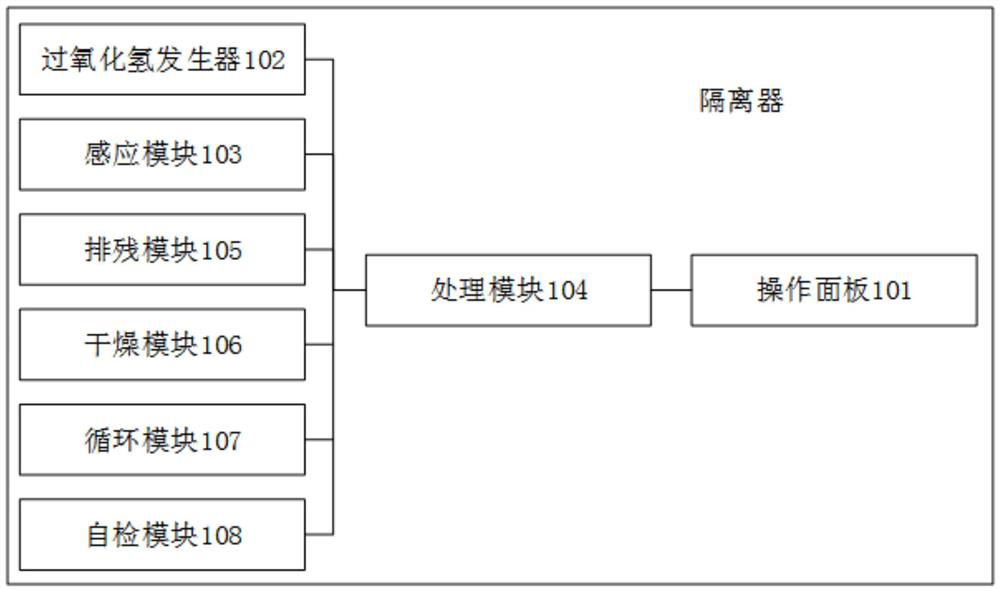
[0066] refer to figure 1 , is an isolator disclosed in this application, specifically including:
[0067] The operation panel 101 is used to receive the sterilization instruction input by the experimenter;
[0068] Hydrogen peroxide generator 102, for generating hydrogen peroxide gas, and passing hydrogen peroxide gas into the isolator;
[0069] The processing module 104 is used to control the hydrogen peroxide generator 102 to control the hydrogen peroxide gas concentration in the isolator according to the sterilization instruction, so that the hydrogen peroxide gas completes the sterilization process inside the isolator;
[0070] The sensing module 103 is used for real-time monitoring and feeding back the concentration of hydrogen peroxide gas in the isolator to the processing module 104;
[0071] The residue removal module 105 is used to remove the hydrogen peroxide gas in the isolator after the hydrogen peroxide gas sterilizes the inside of the isolator;
[0072] Among ...
Embodiment 2
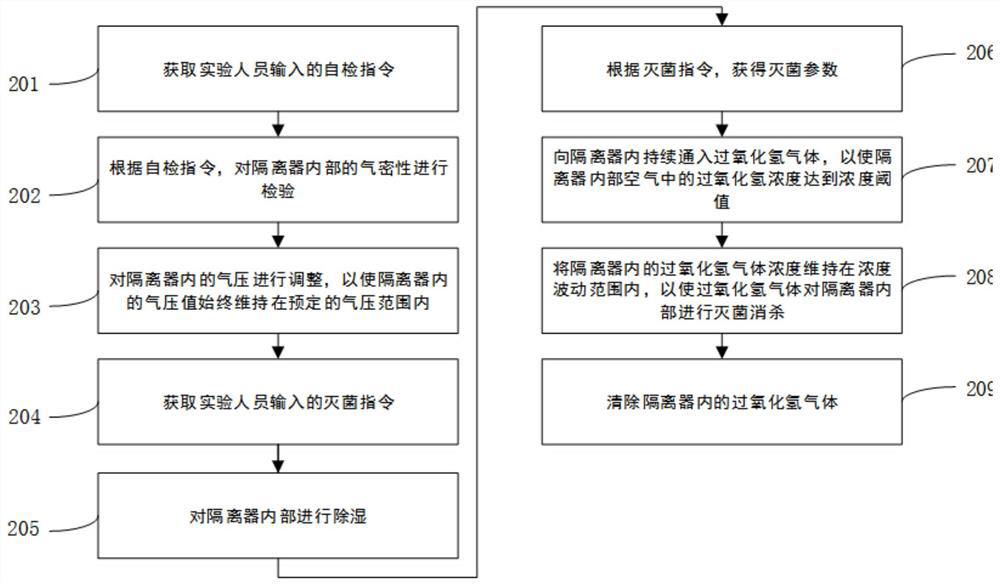
[0114] refer to image 3 , is a hydrogen peroxide gas sterilization method disclosed in the present application. This embodiment is applied to the isolator described in Embodiment 1. The method described in this embodiment specifically includes:
[0115] 201. Obtain a self-inspection instruction input by an experimenter.
[0116] 202. According to the self-inspection instruction, the airtightness inside the isolator is inspected.
[0117] Specifically, A1. After closing the hatch of the isolator, obtain the self-inspection parameters according to the self-inspection instruction. The self-inspection parameters include at least positive pressure threshold, voltage stabilization time limit, self-inspection time limit and qualified loss;
[0118] The positive pressure threshold above is used to illustrate the positive pressure value that the isolator needs to achieve during self-test;
[0119] The above voltage stabilization time limit is used to explain the time limit for the i...
Embodiment 3
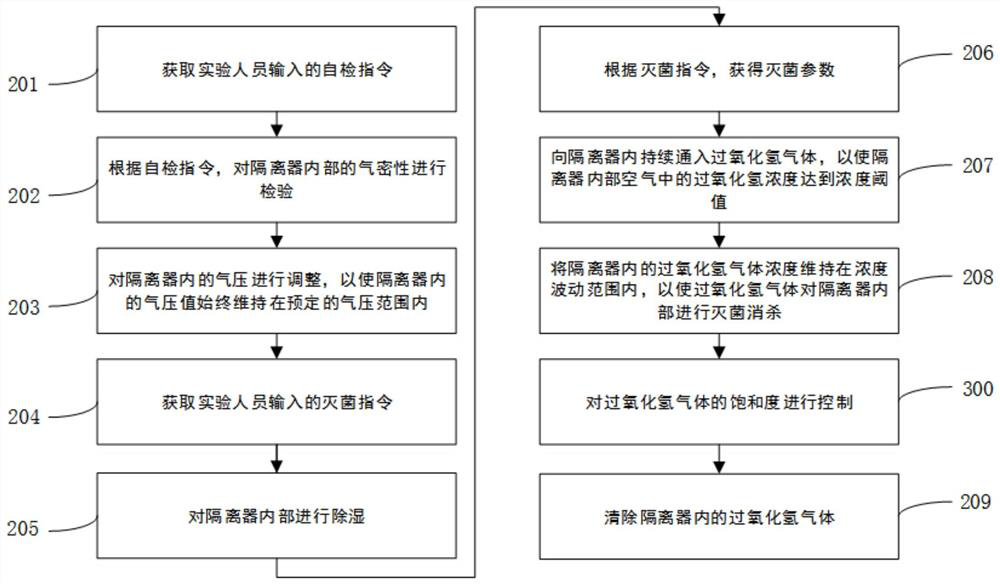
[0165] refer to Figure 4 , is a hydrogen peroxide gas sterilization method disclosed in this application. This embodiment is based on the scheme of Embodiment 2, and has been optimized and improved. In particular, the saturation threshold, the second Sterilization time limit, upper limit of saturation and lower limit of saturation; at the same time, during the execution of step 208, step 300 is executed synchronously, and the improved part will be further elaborated below:
[0166] The saturation threshold above is used to illustrate the optimum saturation for hydrogen peroxide gas sterilization;
[0167] The above-mentioned second sterilization time limit is used to illustrate that hydrogen peroxide gas needs to maintain the minimum time limit of the saturation threshold to achieve the expected sterilization effect. The second sterilization time limit is shorter than the first sterilization time limit mentioned in Example 1. Bacteria time limit;
[0168] The above upper li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com