Repotrectinib crystal form and preparation method thereof
A crystal form and selected technology, applied in the field of medicinal chemistry, can solve problems such as differences in drug solubility, stability and fluidity, different clinical effects, and effects on drug safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Embodiment 1: Preparation of crystal form CM-I
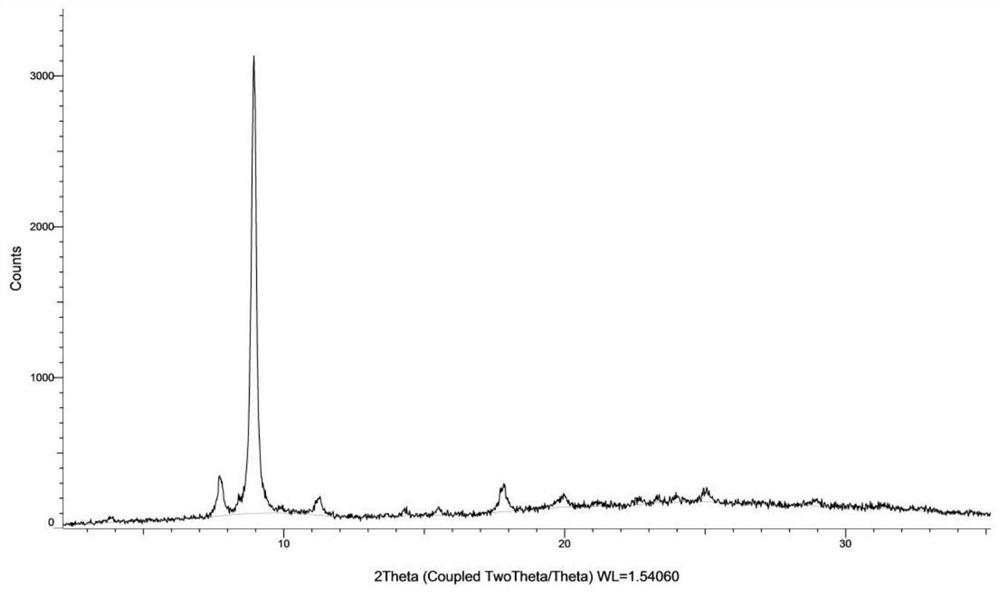
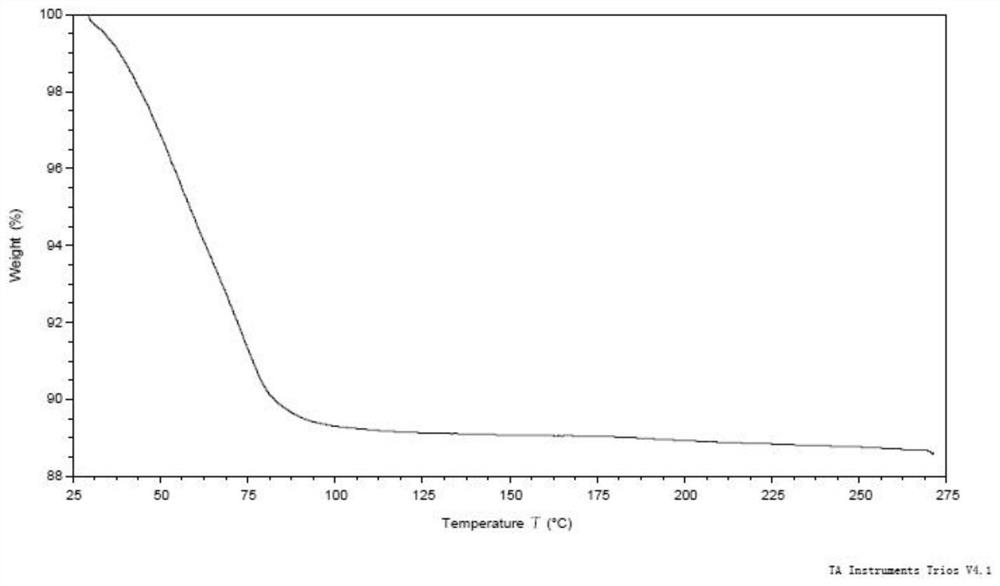
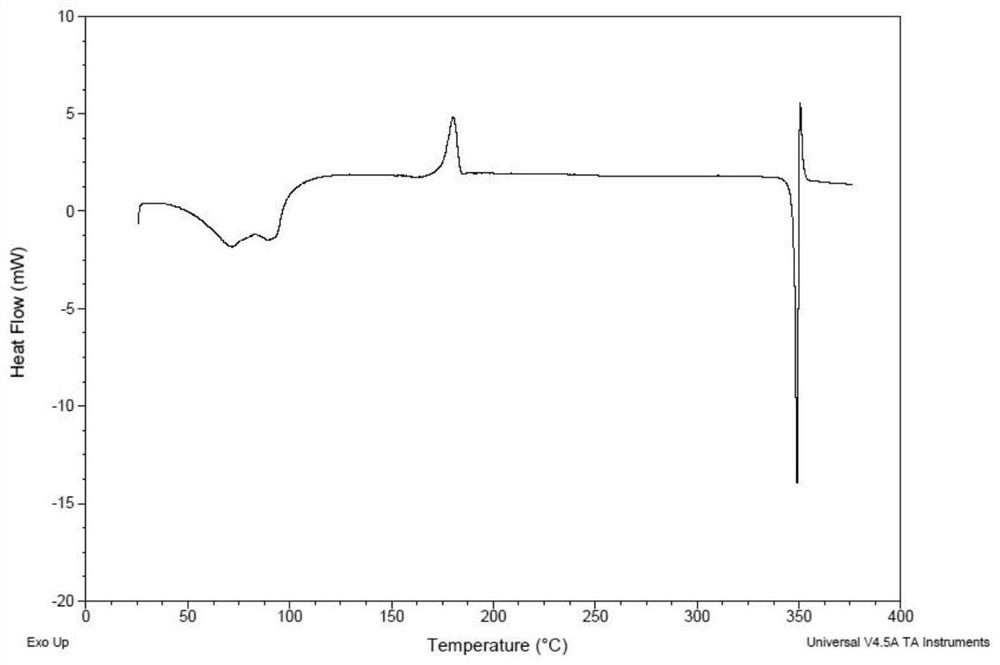
[0135] Weigh 500 mg of the compound of formula (I), dissolve it in 27 mL of ethanol at 50° C., and filter. The filtrate was added dropwise to 200mL water at 20°C, stirred for 2h, and filtered. The wet filter cake was vacuum-dried at 25°C for 24 hours, and the obtained solid was the crystal form CM-I of the compound of formula (I). Carry out XRPD test to the obtained solid, its X-ray powder diffraction data are as shown in table 1, and its XRPD pattern is as follows figure 1 Shown; Carry out TGA test to gained solid, its spectrogram is as figure 2 As shown, the weight loss is about 10.5% at 25-100°C; DSC test is performed on the obtained solid, and there are two endothermic peaks at 60°C-92°C, and one exothermic peak at 170°C-185°C. image 3 Shown; Gained solid is carried out 1H NMR test, and its spectrogram is as Figure 4 Shown, NMR data: 1H NMR (400MHz, DMSO-d 6)δ9.83(s,1H),8.81(d,J=6.7Hz,1H),8.58(d,J=7.6Hz,1H),8....
Embodiment 2
[0138] Embodiment 2: the preparation of crystal form CM-I
[0139] Weigh 9mg of the compound of formula (I), dissolve it in 0.4mL formic acid / water (4:1, v / v) at room temperature, and filter. The filtrate was added dropwise to 4 mL of water at 28 °C, stirred for 2 h, and filtered. The solid was dried under vacuum at 25°C for 24 hours, and the obtained solid was the crystal form CM-I of the compound of formula (I). The obtained solid was tested by XRPD, and its X-ray powder diffraction data are shown in Table 2.
[0140] Table 2
[0141] 2θ(°) Relative Strength(%) 2θ(°) Relative Strength(%) 3.7 1.9 17.6 4.2 7.5 11.4 20.0 3.2 8.7 100.0 24.9 2.4 11.1 3.8 28.7 1.7
Embodiment 3
[0142] Embodiment 3: the preparation of crystal form CM-I
[0143] Weigh 10 mg of the compound of formula (I), dissolve it in 0.5 mL of tetrahydrofuran at room temperature, and filter. Slowly drop 1mL of water into the filtrate, stir for 2h, and filter. The solid was dried under vacuum at 25°C for 24 hours, and the obtained solid was the crystal form CM-I of the compound of formula (I). The obtained solid was tested by XRPD, and its X-ray powder diffraction data are shown in Table 3.
[0144] table 3
[0145] 2θ(°) Relative Strength(%) 2θ(°) Relative Strength(%) 7.6 8.0 15.3 2.3 8.7 100.0 17.6 5.7 11.1 3.6 19.8 3.1 14.2 1.6 24.9 3.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com