Pharmaceutical composition containing favipiravir as well as preparation method and application thereof
A technology for favipiravir and composition, which is applied in the field of pharmaceutical composition containing favipiravir and its preparation, can solve the problem of improving the potential risk of favipiravir transformation, increasing production cost, and being unfavorable to guarantee the quality of pharmaceutical preparations Uniformity, effectiveness, safety and quality control issues, to achieve the effect of controllable drug quality, improve production efficiency, and reduce the risk of crystal transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
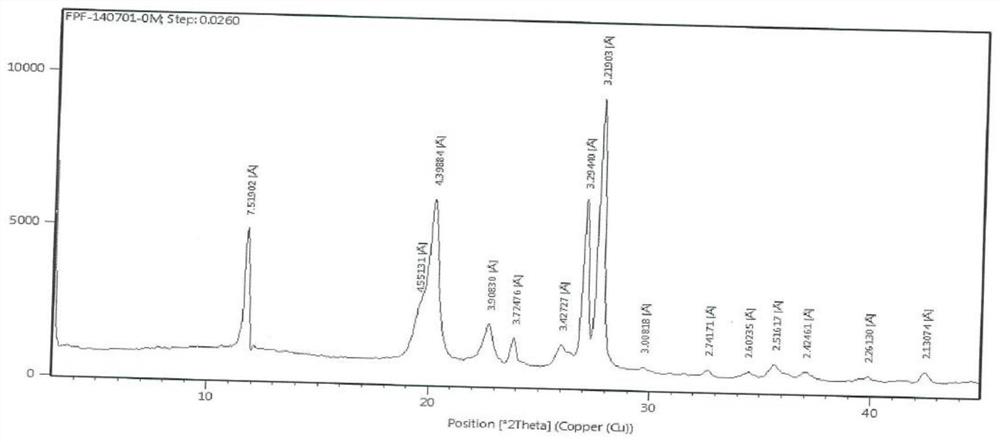
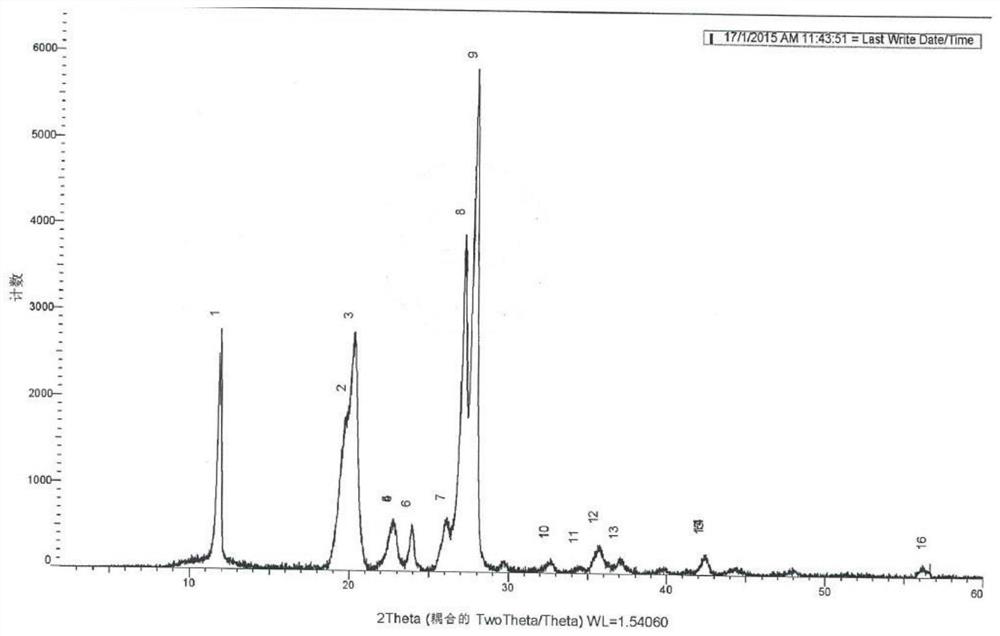
Image
Examples
Embodiment 1-3
[0135] Example 1-3 Preparation of Favipiravir Tablets
[0136] The Favipiravir tablet composition of embodiment 1-3 is shown in Table 1, and its preparation method comprises the following steps:
[0137] (1) Pretreatment of raw and auxiliary materials: Favipiravir is pulverized and used for standby; Crospovidone XL is pulverized and passed through an 80-mesh sieve for subsequent use; colloidal silicon dioxide is passed through a 60-mesh sieve for subsequent use.
[0138] (2) Adhesive preparation: take by weighing the povidone K30 of prescription quantity, add appropriate amount of purified water, stir and dissolve, it is mixed with the aqueous solution of 20% povidone K30.
[0139] (3) premixing: take by weighing Favipiravir, colloidal silicon dioxide and crospovidone XL of prescription quantity, place in the dry grinding cup, stir (speed 400r / min), shear (speed 800r / min) / min) for 5min to obtain a premixed powder.
[0140] (4) Granulation: add the prescribed amount of bin...
Embodiment 4-6
[0148] Example 4-6 Tablet Performance and Dissolution Investigation
[0149] 1. Tablet performance investigation
[0150] Investigate embodiment 1-3 tablet performance, the results are shown in Table 2.
[0151] Table 2 embodiment 1-3 tablet performance
[0152]
[0153] From the results in Table 2, it can be seen that the granules prepared in Examples 1-3 are uniform, have good fluidity and compressibility, are not sticky during the tableting process, have a smooth surface, stable tablet weight, and meet the requirements for hardness and friability.
[0154] 2. Dissolution Determination
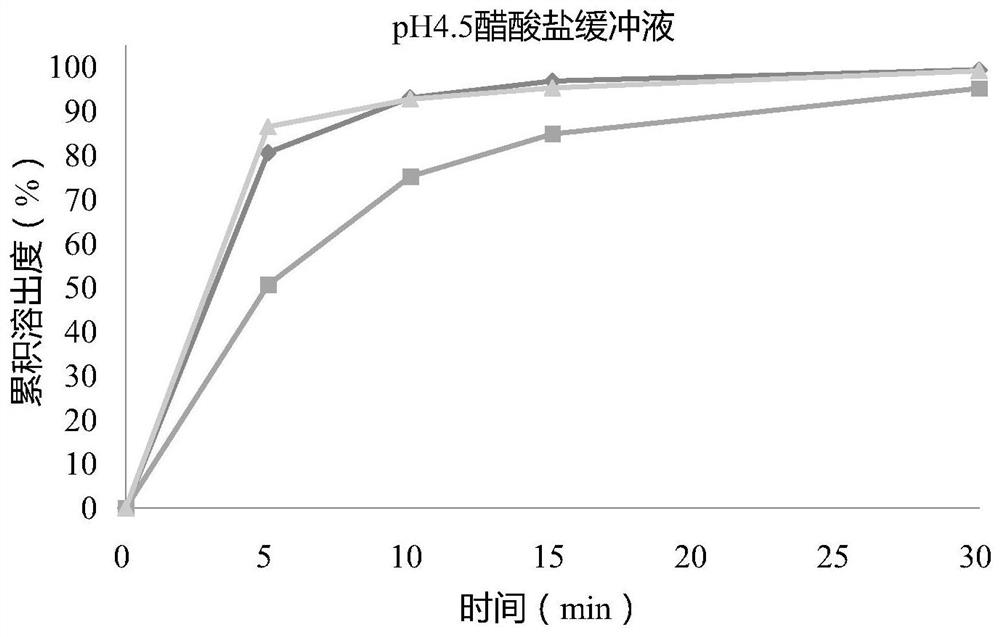
[0155] Adopt the second method paddle method, 50rpm / min, with pH4.5 acetate buffer 900mL as dissolution medium, investigate embodiment 1-3 tablet stripping performance, the results are shown in Table 3 and attached figure 1 .
[0156] Table 3 Example 1-3 Tablet Dissolution Rate (n=6)
[0157]
[0158] From Table 3 and attached figure 1 It can be seen from the results that the t...
Embodiment 3
[0161] Table 4 embodiment 3 tablet stability
[0162]
[0163] As can be seen from the results in Table 4, after the tablet of Example 3 was placed for 10 days under high temperature (60° C.), high humidity (RH92.5%), and light conditions of 5000 lx, the sample weight increased slightly in 10 days of high humidity (RH92.5%) , After the detection of related substances, compared with the 0 day, each individual impurity and total impurity (%) had no significant change, which met the standard limit requirements.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com