Fasotinib crystal form and preparation method thereof
A crystal form and crystallization technology, which is applied in organic chemical methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems affecting drug safety and effectiveness, drug solubility, stability and fluidity differences, clinical different effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1: Preparation of crystal form XM-I
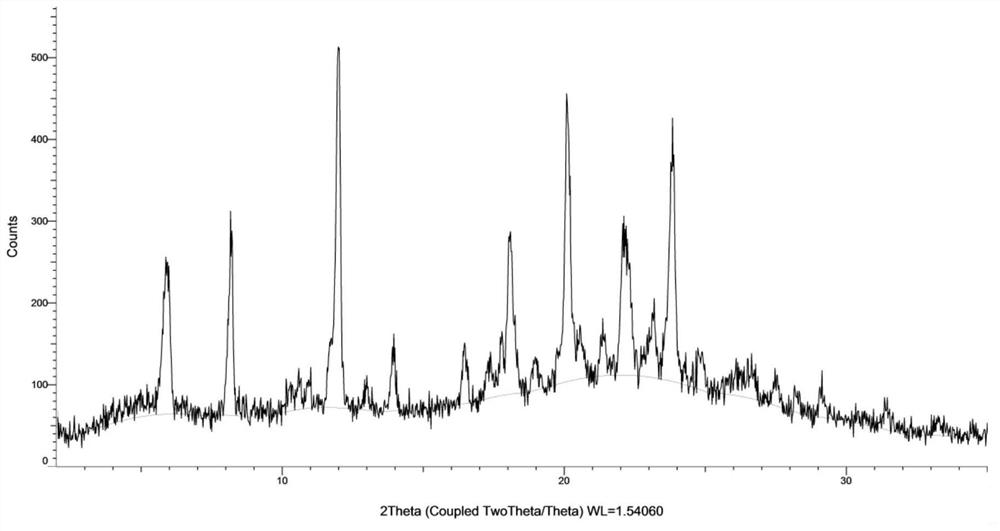
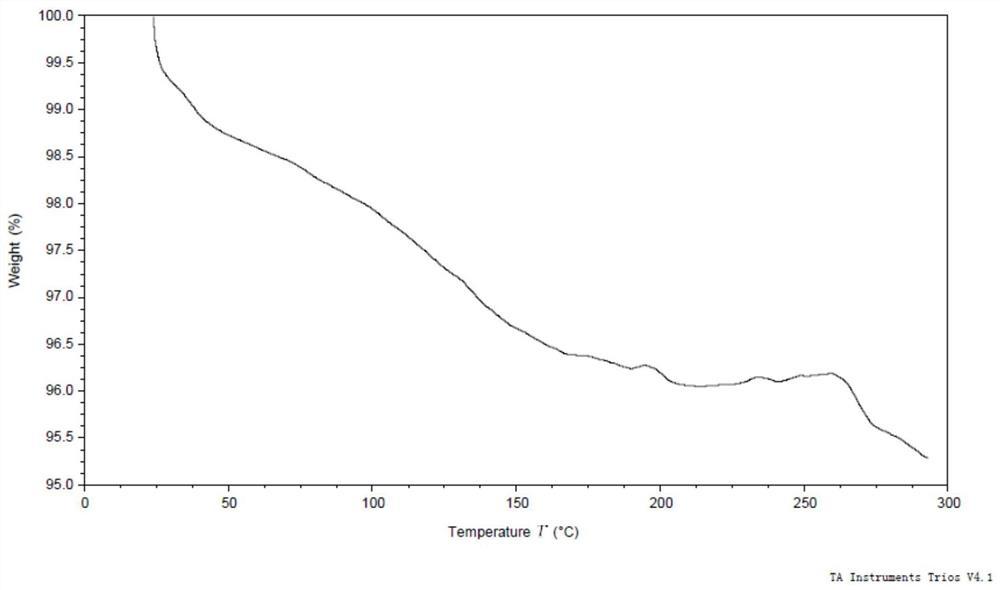
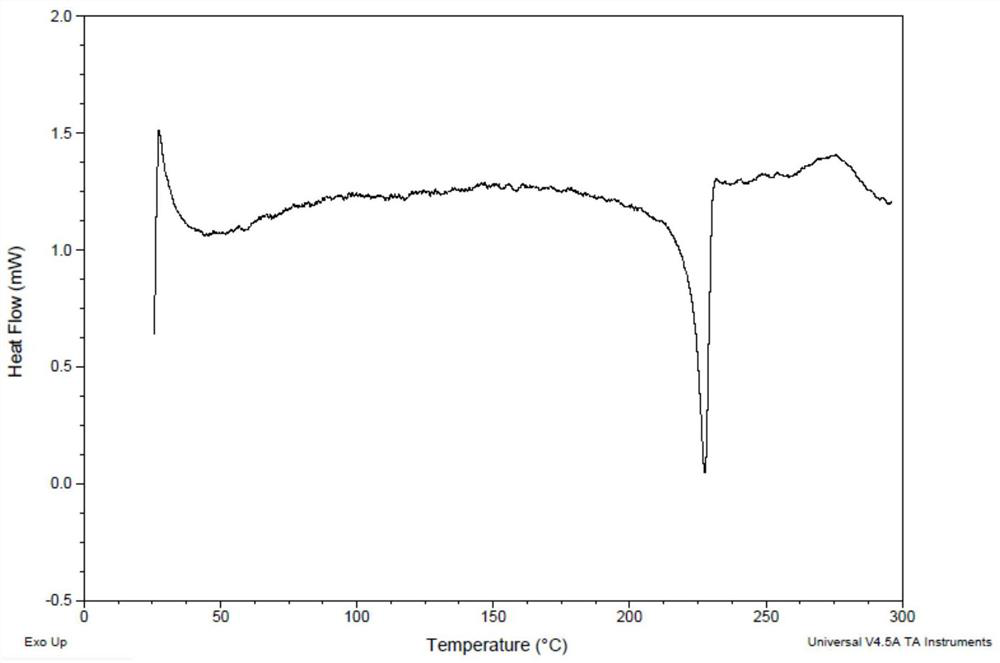
[0106] The 50 mg of formula (I) compound was sunedressed in 1 ml of 2-butanone, filtered, and 3 ml of petroleum ether was slowly added to the filtrate, stirred at 25 ° C for 16 h, and there was solid precipitation, the resulting solid was formula (I) Compound type XM -I. The obtained solid was performed, and the X-ray powder diffraction data was shown in Table 1, and its XRPD is shown in Table 1. figure 1 Down; TGA testing of the resulting solid, whose spectrum is figure 2 Down; DSC testing of the resulting solid, whose spectrum is image 3 Disted; for the resulting solid 1 H NMR test, whose spectrum is Figure 4 As shown, nuclear magnetic data: 1 H NMR (400MHz, DMSO-D 6 Δ9.18 (S, 1H), 8.01 (D, J = 7.1 Hz, 1H), 7.69 (S, 1H), 7.55-7.47 (M, 2H), 7.01 (S, 2H), 6.25 (DD, J = 17.1, 10.2 Hz, 1H), 6.06 (DD, J = 17.1, 2.1 Hz, 1H), 5.55 (DD, J = 10.2, 2.1 Hz, 1H), 4.34 (S, 2H), 3.97 (s, 6h) 3.85 (D, J = 11.6Hz, 2H), 3.65 (D, J = 10.4 Hz, 1...
Embodiment 2
[0110] Example 2: Preparation of crystalline XM-I
[0111] It is weighed with 50 mg of the compound of formula (I) to dissolve in 1 ml of acetonitrile, filtered, and 15 ml of diethyl ether was slowly added to the filtrate, stirred at 25 ° C for 16 h, there was solid precipitation, the resulting solid was formula (I) Compound crystal type XM-I, obtained The solid is the formula (I) Compound crystal type XM-I. The obtained solid was performed, and the X-ray powder diffraction data was shown in Table 2.
[0112] Table 2
[0113]
[0114]
Embodiment 3
[0115] Example 3: Preparation of crystalline XM-I
[0116] It is weighted 15 mg of the compound of formula (I) in 0.2 ml of ethanol / n-heptane (1: 4, v / v) at 40 ° C, filtered, stirred at 5 ° C for 16 h, there is solid precipitation, resulting solid For the formula (I) compound crystal type XM-I. The obtained solid is performed, and the X-ray powder diffraction data is shown in Table 3.
[0117] table 3
[0118]
[0119]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com