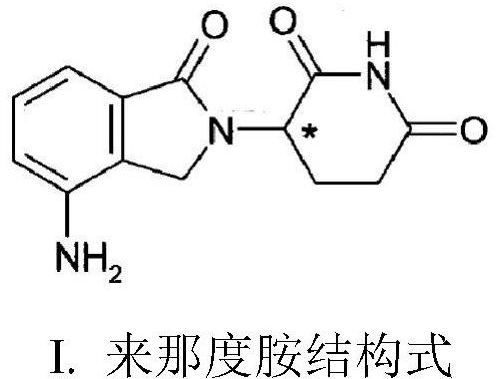
Lenalidomide pharmaceutical composition and preparation method therefor
A technology of lenalidomide and its origin is applied in the field of lenalidomide pharmaceutical composition and its preparation, which can solve the problems such as the increase of impurities in solid oral preparations, complicated process, influence on drug dissolution, etc., to reduce the risk of crystal transformation and to achieve a simple process. Environmental protection, stable and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
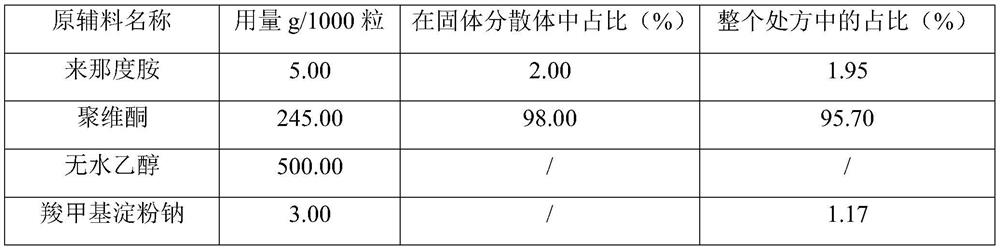
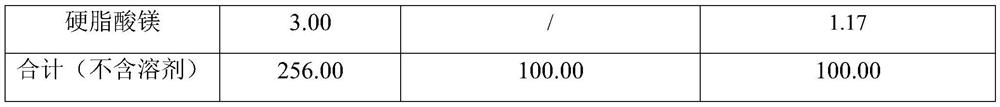
[0029] Example 1: Lenalidomide pharmaceutical composition prescription composition: specification 5mg, capsules, prescription quantity is 1000 capsules (see Table 1).
[0030] Table 1: Example 1 prescription table
[0031]
[0032]
[0033] The preparation method is as follows:
[0034] (1) Dissolve the prescribed amount of lenalidomide and povidone in absolute ethanol, and dry at 60°C using a double-drum dryer;
[0035] (2) The scraped solid dispersion adopts 24 mesh sieves to granulate;
[0036] (3) Add the solid dispersion, sodium carboxymethyl starch and magnesium stearate into the mixer, set the speed at 10 rpm, and mix for 5 minutes;
[0037] (4) Fill in No. 2 gelatin hollow capsules.
Embodiment 2
[0038] Example 2: Lenalidomide pharmaceutical composition Prescription composition: specification 25mg, capsules, prescription quantity is 1000 capsules (see Table 2).
[0039] Table 2: Example 2 prescription table
[0040] Name of raw material Dosage g / 1000 capsules Proportion in solid dispersion (%) Proportion in the entire prescription (%) Lenalidomide 25.00 40.00 38.76 Povidone 37.50 60.00 58.14 Isopropanol 100.00 / / Croscarmellose Sodium 1.00 / 1.55 Magnesium stearate 1.00 / 1.55 Total (without solvent) 64.50 100.00 100.00
[0041] The preparation method is the same as in Example 1, only the above-mentioned ingredients are replaced, and the gelatin hollow capsule in step (4) is replaced with No. 3.
Embodiment 3
[0042] Example 3: Lenalidomide pharmaceutical composition prescription composition: specification 25mg, tablet, prescription quantity is 1000 tablets (see Table 3).
[0043] Table 3: Example 3 prescription table
[0044]
[0045]
[0046] Preparation:
[0047] (1) Dissolve the prescribed amount of lenalidomide and polyethylene glycol 4000 in isopropyl ketone, and dry at 60°C with a double-drum dryer;
[0048] (2) The scraped solid dispersion adopts 24 mesh sieves to granulate;
[0049] (3) Add the solid dispersion, crospovidone and magnesium stearate into the mixer, set the rotation speed at 10 rpm, and mix for 5 minutes;
[0050] (4) Tablet pressing is carried out with a tablet press.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com