Crystal form of salt of sb-939 and its preparation method and use
A crystal form, CS1 technology, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, drug combinations, etc., to achieve the effect of low moisture absorption, good stability, consistent and controllable quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~2
[0134] Embodiment 1-2: Preparation of crystal form CS7
Embodiment 1
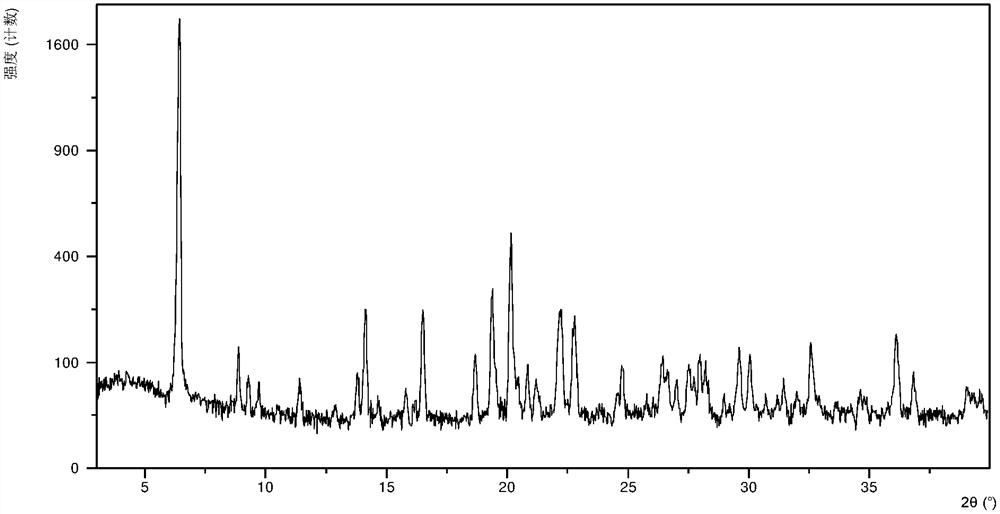
[0136] Weigh 200.1 mg of SB-939 free base solid, add it to 2.5 ml of a mixed system of isopropanol and isopropyl acetate with a volume ratio of 1:1, stir and slowly add 200 microliters of 6 mol / liter hydrochloric acid aqueous solution dropwise , stirred at room temperature for 24 h, the solid was collected by centrifugation, and the crystal form CS7 was obtained after vacuum drying. The crystal form CS7 is a hydrate, and its X-ray powder diffraction pattern is as follows: figure 1 , X-ray powder diffraction data are shown in Table 1.
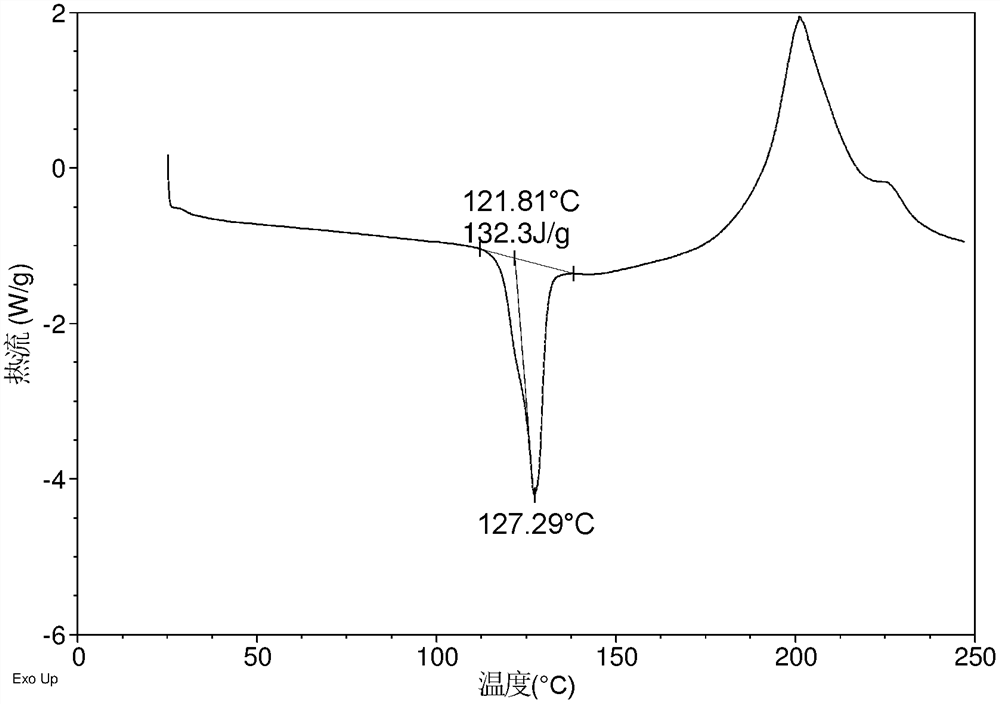
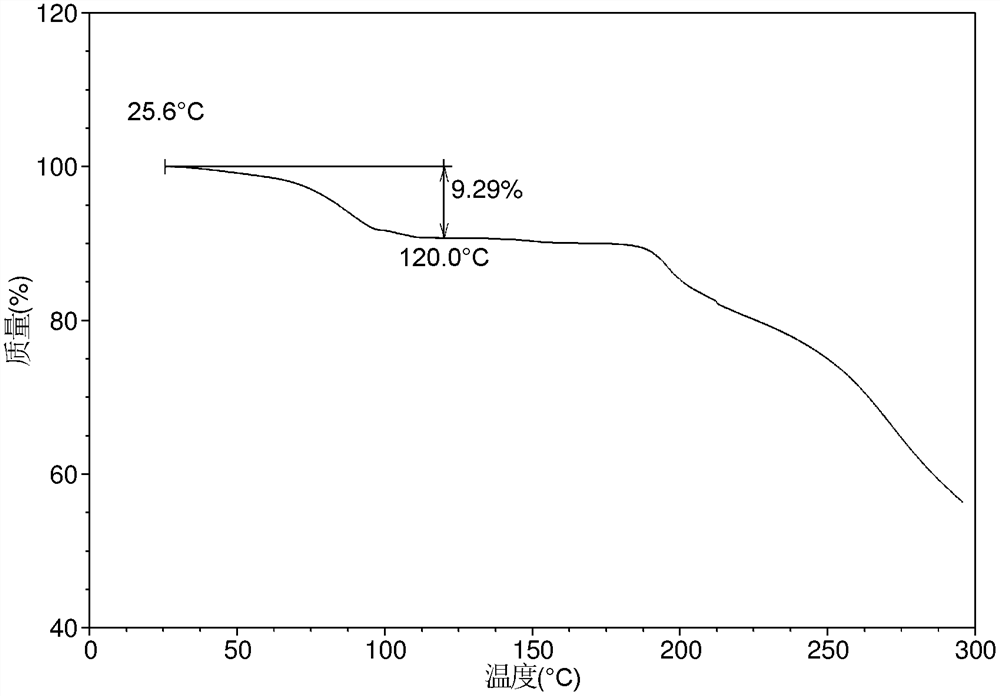
[0137] The DSC curve of the crystal form CS7 obtained in embodiment 1 is as follows figure 2 As shown, the TGA curve is as image 3 shown.
[0138] Table 1
[0139]
[0140]
Embodiment 2
[0142] Weigh 10.3 mg of SB-939 dihydrochloride raw material, dissolve it in 0.3 ml of water at room temperature, stir and quickly add 3 ml of a mixed solvent system of isopropanol and ethyl acetate with a volume ratio of 1:1, and continue stirring Until a large amount of solids are precipitated, the solids are collected by centrifugation to obtain crystal form CS7, and its X-ray powder diffraction pattern is as follows: Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com