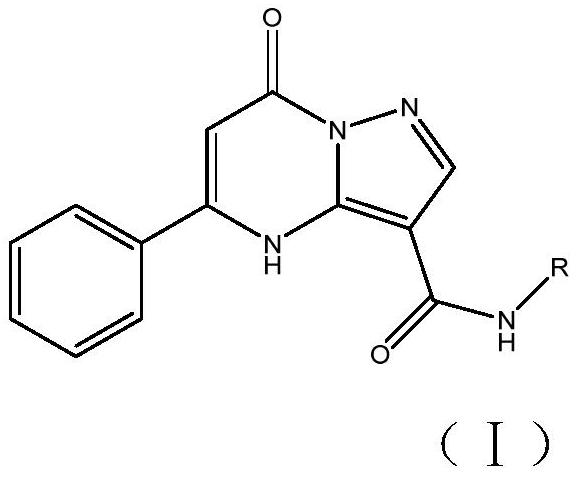
A kind of PDE2 inhibitor phenylpyrazolopyrimidine compound and preparation method thereof
A technology of phenylpyrazolopyrimidine and compound, applied in the field of medicinal chemistry, can solve the problem of low bioavailability in vivo, achieve excellent pharmacodynamic performance, good PDE2 inhibitory activity, and meet the requirements of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
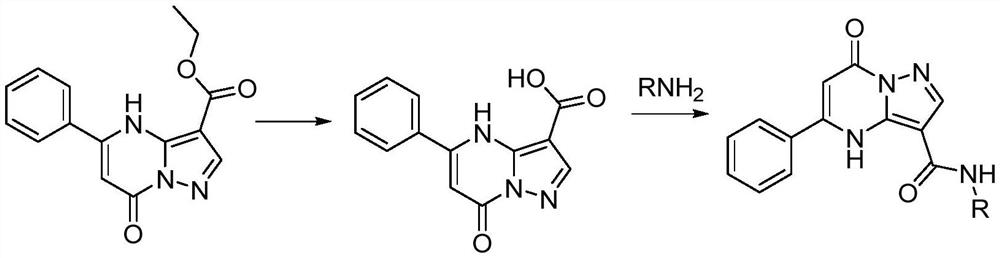
[0030] Embodiment 1: the preparation method of compound a
[0031] (1) Synthesis of 7-oxo-5-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine-3-carboxylic acid
[0032]
[0033] Weigh 1.2 g of beige solid powder 7-oxo-5-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine-3-carboxylate 1.2 g into a 250 mL round bottom flask, first Add 32 mL of EtOH, and the temperature is 20°C. After stirring for 5 min, add 16 mL of THF, and then slowly add 40 mL of water. After the temperature rose to 45°C, 570 mg of KOH was added, and the solid was almost completely dissolved after stirring for 10 min. Maintain the temperature at 50°C for the reaction. After 3 hours, 350 mg of KOH was weighed again and added to the reaction solution, and the temperature was raised to 80° C., at which point the solution was basically clear. After 3 hours, weigh 200 mg of KOH and add to the reaction system, reflux, stop the reaction for 12 hours, the solution is light pink, cool to room temperature, slowly add 1mol / L H...
Embodiment 2
[0037] Embodiment 2: the preparation method of compound b
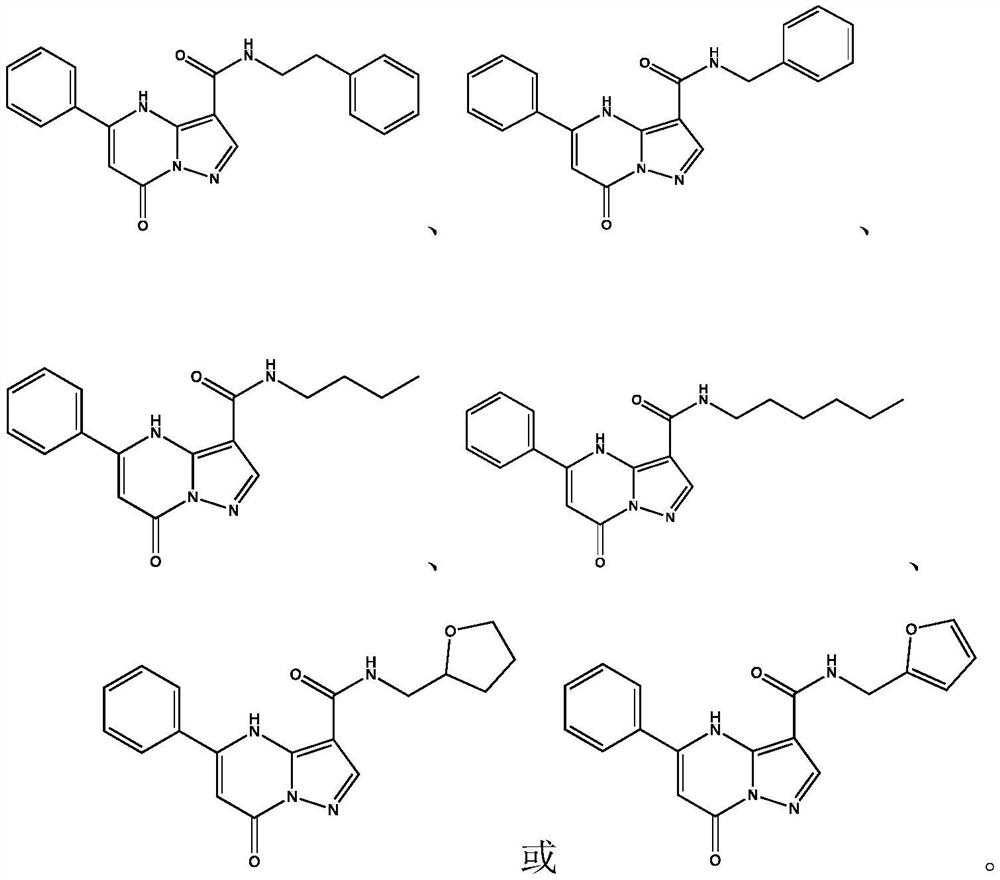
[0038] Except that benzylamine was used instead of phenethylamine, the rest of the synthesis steps were the same as the synthesis method of compound a in Example 1. Synthesis of N-Benzyl-7-oxo-5-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine-3-carboxamide
[0039]
[0040] The purity detected by HPLC was 98.12%, and the yield was 49.4%. 1H NMR(400MHz,DMSO-d6)δ9.08(s,1H),8.01(s,1H),7.91–7.82(m,2H),7.39–7.32(m,8H),6.10(s,1H), 4.56(s,2H).
Embodiment 3
[0041] Embodiment 3: the preparation method of compound c
[0042] Except that n-butylamine was used instead of phenethylamine, the rest of the synthesis steps were the same as the synthesis method of compound a in Example 1. Synthesis of N-butyl-7-oxo-5-phenyl-4,7-dihydropyrazolo[1,5-a]pyrimidine-3-carboxamide
[0043]
[0044] The purity detected by HPLC was 97.62%, and the yield was 54.8%. 1 HNMR(400MHz,DMSO-d6)δ8.70(s,1H),7.98(d,J=7.4Hz,2H),7.93(s,1H),7.52–7.34(m,3H),6.07(s,1H ), 3.32 (t, J = 10.0Hz, 2H), 1.59–1.49 (m, 2H), 1.43–1.40 (m, 2H), 0.93 (t, J = 7.2Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com